Role of double-stranded DNA translocase activity of human HLTF in replication of damaged DNA
- PMID: 19948885
- PMCID: PMC2812231
- DOI: 10.1128/MCB.00863-09
Role of double-stranded DNA translocase activity of human HLTF in replication of damaged DNA
Abstract
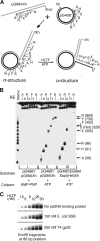
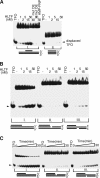
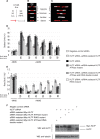
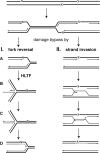
Unrepaired DNA lesions can block the progression of the replication fork, leading to genomic instability and cancer in higher-order eukaryotes. In Saccharomyces cerevisiae, replication through DNA lesions can be mediated by translesion synthesis DNA polymerases, leading to error-free or error-prone damage bypass, or by Rad5-mediated template switching to the sister chromatid that is inherently error free. While translesion synthesis pathways are highly conserved from yeast to humans, very little is known of a Rad5-like pathway in human cells. Here we show that a human homologue of Rad5, HLTF, can facilitate fork regression and has a role in replication of damaged DNA. We found that HLTF is able to reverse model replication forks, a process which depends on its double-stranded DNA translocase activity. Furthermore, from analysis of isolated dually labeled chromosomal fibers, we demonstrate that in vivo, HLTF promotes the restart of replication forks blocked at DNA lesions. These findings suggest that HLTF can promote error-free replication of damaged DNA and support a role for HLTF in preventing mutagenesis and carcinogenesis, providing thereby for its potential tumor suppressor role.
Figures


References
-
- Cordeiro-Stone, M., A. M. Makhov, L. S. Zaritskaya, and J. D. Griffith. 1999. Analysis of DNA replication forks encountering a pyrimidine dimer in the template to the leading strand. J. Mol. Biol. 289:1207-1218. - PubMed
-
- Fishel, R., M. K. Lescoe, M. R. Rao, N. G. Copeland, N. A. Jenkins, J. Garber, M. Kane, and R. Kolodner. 1993. The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell 75:1027-1038. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
