Gene deletion mutants reveal a role for semaphorin receptors of the plexin-B family in mechanisms underlying corticogenesis
- PMID: 19948886
- PMCID: PMC2812242
- DOI: 10.1128/MCB.01458-09
Gene deletion mutants reveal a role for semaphorin receptors of the plexin-B family in mechanisms underlying corticogenesis
Abstract
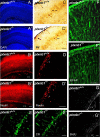
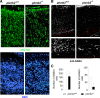
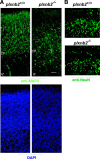
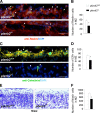
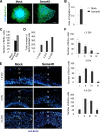
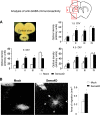
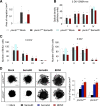
Semaphorins and their receptors, plexins, are emerging as key regulators of various aspects of neural and nonneural development. Semaphorin 4D (Sema4D) and B-type plexins demonstrate distinct expression patterns over critical time windows during the development of the murine neocortex. Here, analysis of mice genetically lacking plexin-B1 or plexin-B2 revealed the significance of Sema4D-plexin-B signaling in cortical development. Deficiency of plexin-B2 resulted in abnormal cortical layering and defective migration and differentiation of several subtypes of cortical neurons, including Cajal-Retzius cells, GABAergic interneurons, and principal cells in vivo. In contrast, a lack of plexin-B1 did not impact on cortical development in vivo. In various ex vivo assays on embryonic forebrain, Sema4D enhanced the radial and tangential migration of developing neurons in a plexin-B2-dependent manner. These results suggest that Sema4D-plexin-B2 interactions regulate mechanisms underlying cell specification, differentiation, and migration during corticogenesis.
Figures






Similar articles
-
The semaphorin 4D-plexin-B signalling complex regulates dendritic and axonal complexity in developing neurons via diverse pathways.Eur J Neurosci. 2009 Oct;30(7):1193-208. doi: 10.1111/j.1460-9568.2009.06934.x. Epub 2009 Sep 29. Eur J Neurosci. 2009. PMID: 19788569
-
Plexin-B family members demonstrate non-redundant expression patterns in the developing mouse nervous system: an anatomical basis for morphogenetic effects of Sema4D during development.Eur J Neurosci. 2004 May;19(10):2622-32. doi: 10.1111/j.0953-816X.2004.03401.x. Eur J Neurosci. 2004. PMID: 15147296
-
Plexin-B2, but not Plexin-B1, critically modulates neuronal migration and patterning of the developing nervous system in vivo.J Neurosci. 2007 Jun 6;27(23):6333-47. doi: 10.1523/JNEUROSCI.5381-06.2007. J Neurosci. 2007. PMID: 17554007 Free PMC article.
-
Roles of Sema4D and Plexin-B1 in tumor progression.Mol Cancer. 2010 Sep 21;9:251. doi: 10.1186/1476-4598-9-251. Mol Cancer. 2010. PMID: 20858260 Free PMC article. Review.
-
Plexins: Navigating through the neural regulation and brain pathology.Neurosci Biobehav Rev. 2025 Feb;169:105999. doi: 10.1016/j.neubiorev.2024.105999. Epub 2025 Jan 3. Neurosci Biobehav Rev. 2025. PMID: 39756719 Review.
Cited by
-
Plexin-B2 regulates the proliferation and migration of neuroblasts in the postnatal and adult subventricular zone.J Neurosci. 2012 Nov 21;32(47):16892-905. doi: 10.1523/JNEUROSCI.0344-12.2012. J Neurosci. 2012. PMID: 23175841 Free PMC article.
-
Integrative mechanisms of oriented neuronal migration in the developing brain.Annu Rev Cell Dev Biol. 2013;29:299-353. doi: 10.1146/annurev-cellbio-101512-122400. Epub 2013 Aug 7. Annu Rev Cell Dev Biol. 2013. PMID: 23937349 Free PMC article. Review.
-
Identification of a calmodulin-binding domain in Sema4D that regulates its exodomain shedding in platelets.Blood. 2013 May 16;121(20):4221-30. doi: 10.1182/blood-2012-11-470609. Epub 2013 Apr 5. Blood. 2013. PMID: 23564909 Free PMC article.
-
Genetic dissection of plexin signaling in vivo.Proc Natl Acad Sci U S A. 2014 Feb 11;111(6):2194-9. doi: 10.1073/pnas.1308418111. Epub 2014 Jan 27. Proc Natl Acad Sci U S A. 2014. PMID: 24469813 Free PMC article.
-
Plexin-mediated neuronal development and neuroinflammatory responses in the nervous system.Histol Histopathol. 2023 Nov;38(11):1239-1248. doi: 10.14670/HH-18-625. Epub 2023 Apr 25. Histol Histopathol. 2023. PMID: 37170703 Review.
References
-
- Allendoerfer, K. L., and C. J. Shatz. 1994. The subplate, a transient neocortical structure: its role in the development of connections between thalamus and cortex. Annu. Rev. Neurosci. 17:185-218. - PubMed
-
- Anton, E. S., M. A. Marchionni, K. F. Lee, and P. Rakic. 1997. Role of GGF/neuregulin signaling in interactions between migrating neurons and radial glia in the developing cerebral cortex. Development 124:3501-3510. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
