Strong nanocomposites with Ca, PO(4), and F release for caries inhibition
- PMID: 19948941
- PMCID: PMC3056546
- DOI: 10.1177/0022034509351969
Strong nanocomposites with Ca, PO(4), and F release for caries inhibition
Abstract
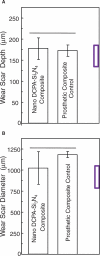
This article reviews recent studies on: (1) the synthesis of novel calcium phosphate and calcium fluoride nanoparticles and their incorporation into dental resins to develop nanocomposites; (2) the effects of key microstructural parameters on Ca, PO(4), and F ion release from nanocomposites, including the effects of nanofiller volume fraction, particle size, and silanization; and (3) mechanical properties of nanocomposites, including water-aging effects, flexural strength, fracture toughness, and three-body wear. This article demonstrates that a major advantage of using the new nanoparticles is that high levels of Ca, PO(4), and F release can be achieved at low filler levels in the resin, because of the high surface areas of the nanoparticles. This leaves room in the resin for substantial reinforcement fillers. The combination of releasing nanofillers with stable and strong reinforcing fillers is promising to yield a nanocomposite with both stress-bearing and caries-inhibiting capabilities, a combination not yet available in current materials.
Figures
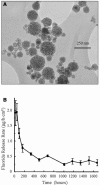

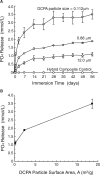
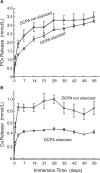

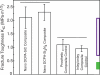
References
-
- Anusavice KJ, Zhang NZ, Shen C. (2005). Effect of CaF2 content on rate of fluoride release from filled resins. J Dent Res 84:440-444 - PubMed
-
- Asmussen E, Peutzfeldt A. (2002). Long-term fluoride release from a glass ionomer cement, a compomer, and from experimental resin composites. Acta Odontol Scand 60:93-97 - PubMed
-
- Bayne SC, Thompson JY, Swift EJ, Jr, Stamatiades P, Wilkerson M. (1998). A characterization of first-generation flowable composites. J Am Dent Assoc 129:567-577 - PubMed
-
- Beun S, Glorieux T, Devaux J, Vreven J, Leloup G. (2007). Characterization of nanofilled compared to universal and microfilled composites. Dent Mater 23:51-59 - PubMed
-
- Braga RR, Ballester RY, Ferracane JL. (2005). Factors involved in the development of polymerization shrinkage stress in resin-composites: a systematic review. Dent Mater 21:962-970 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

