Cobalt and nickel diimine-dioxime complexes as molecular electrocatalysts for hydrogen evolution with low overvoltages
- PMID: 19948953
- PMCID: PMC2791621
- DOI: 10.1073/pnas.0907775106
Cobalt and nickel diimine-dioxime complexes as molecular electrocatalysts for hydrogen evolution with low overvoltages
Abstract
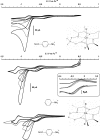
Hydrogen production through the reduction of water appears to be a convenient solution for the long-run storage of renewable energies. However, economically viable hydrogen production requests platinum-free catalysts, because this expensive and scarce (only 37 ppb in the Earth's crust) metal is not a sustainable resource [Gordon RB, Bertram M, Graedel TE (2006) Proc Natl Acad Sci USA 103:1209-1214]. Here, we report on a new family of cobalt and nickel diimine-dioxime complexes as efficient and stable electrocatalysts for hydrogen evolution from acidic nonaqueous solutions with slightly lower overvoltages and much larger stabilities towards hydrolysis as compared to previously reported cobaloxime catalysts. A mechanistic study allowed us to determine that hydrogen evolution likely proceeds through a bimetallic homolytic pathway. The presence of a proton-exchanging site in the ligand, furthermore, provides an exquisite mechanism for tuning the electrocatalytic potential for hydrogen evolution of these compounds in response to variations of the acidity of the solution, a feature only reported for native hydrogenase enzymes so far.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

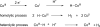

References
-
- Artero V, Fontecave M. Some general principles for designing electrocatalysts with hydrogenase activity. Coordin Chem Rev. 2005;249:1518–1535.
-
- Baffert C, Artero V, Fontecave M. Cobaloximes as functional models for hydrogenases. 2. Proton electroreduction catalyzed by difluoroborylbis(dimethylglyoximato)cobalt(II) complexes in organic media. Inorg Chem. 2007;46:1817–1824. - PubMed
-
- Razavet M, Artero V, Fontecave M. Proton electroreduction catalyzed by cobaloximes: Functional models for hydrogenases. Inorg Chem. 2005;44:4786–4795. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

