Phase I study of intravenous vascular endothelial growth factor trap, aflibercept, in patients with advanced solid tumors
- PMID: 19949018
- PMCID: PMC2815710
- DOI: 10.1200/JCO.2009.22.9237
Phase I study of intravenous vascular endothelial growth factor trap, aflibercept, in patients with advanced solid tumors
Abstract
Purpose: Vascular endothelial growth factor (VEGF) Trap (aflibercept) is an angiogenesis inhibitor comprising portions of the extracellular domains of human VEGF receptors 1 and 2 fused to the Fc portion of human immunoglobulin G. This phase I study was designed to evaluate the safety, pharmacokinetics, and pharmacodynamics of VEGF Trap administered intravenously (IV) every 2 weeks.
Patients and methods: Patients with refractory solid tumors or non-Hodgkin's lymphoma with adequate organ function were eligible. Pharmacokinetic/pharmacodynamic markers included measurement of plasma VEGF bound to VEGF Trap and free VEGF Trap. Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) was incorporated to measure the biologic effects of the drug on tumor vascularity and permeability.
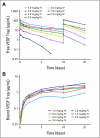
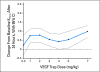
Results: The study enrolled 47 patients at doses ranging from 0.3 to 7.0 mg/kg IV every 2 weeks. Dose-limiting toxicities were rectal ulceration and proteinuria at the 7.0 mg/kg dose. Other mechanism-specific toxicities included hypertension. On the basis of these observations and on pharmacokinetics, the recommended phase II dose of VEGF Trap as a single agent is 4 mg/kg every 2 weeks. Three RECIST (Response Evaluation Criteria in Solid Tumors) -defined partial responses were observed, one at the 3.0 mg/kg and two at the 7.0 mg/kg dose level. Maximum plasma concentration of free VEGF Trap increased proportionally with dose. Maximal VEGF-bound VEGF Trap complex levels were reached at doses > or = 2.0 mg/kg. Changes in volume transfer constant measured by DCE-MRI at baseline and at 24 hours after administration indicate a possible dose-related change in this pharmacodynamic marker.
Conclusion: IV VEGF Trap was well tolerated at the dose levels tested. Pharmacodynamic and pharmacokinetic markers were indicative of VEGF blockade.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Comment in
-
Hunting and trapping the vascular endothelial growth factor.J Clin Oncol. 2010 Jan 10;28(2):185-7. doi: 10.1200/JCO.2009.25.4359. Epub 2009 Nov 30. J Clin Oncol. 2010. PMID: 19949005 No abstract available.
-
Biomarkers of antiangiogenic therapy: how do we move from candidate biomarkers to valid biomarkers?J Clin Oncol. 2010 Jan 10;28(2):183-5. doi: 10.1200/JCO.2009.24.8021. Epub 2009 Nov 30. J Clin Oncol. 2010. PMID: 19949009 No abstract available.
References
-
- Coultas L, Chawengsaksophak K, Rossant J. Endothelial cells and VEGF in vascular development. Nature. 2005;438:937–945. - PubMed
-
- Senger DR, Perruzzi CA, Feder J, et al. A highly conserved vascular permeability factor secreted by a variety of human and rodent tumor cell lines. Cancer Res. 1986;46:5629–5632. - PubMed
-
- Brown LF, Berse B, Jackman RW, et al. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in adenocarcinomas of the gastrointestinal tract. Cancer Res. 1993;53:4727–4735. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

