Cutting edge: the Th1 response inhibits the generation of peripheral regulatory T cells
- PMID: 19949064
- PMCID: PMC2908389
- DOI: 10.4049/jimmunol.0903412
Cutting edge: the Th1 response inhibits the generation of peripheral regulatory T cells
Abstract
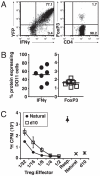
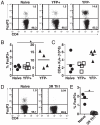
The possibility that effector T cells can be converted into forkhead box P3(+) regulatory T cells (Tregs) has potential therapeutic implications. To analyze the relationship between Th1 effectors and Tregs, we have used a model of systemic autoimmunity in which both effector and Tregs arise from a single population specific for a transgene-encoded systemic protein. In vitro, the presence of IFN-gamma inhibits Treg generation during activation. Using IFN-gamma reporter mice, we demonstrate that IFN-gamma-producing cells tend not to develop into Tregs, and Th1 priming of T cells prior to cell transfer limits the number of forkhead box P3(+) T cells generated in vivo. Moreover, transfer of IFN-gamma(-/-) or STAT1(-/-) T cells resulted in an increase in the number of Tregs. These data support a role for Th1 effector molecules and transcription factors in the control of peripheral Treg generation and demonstrates the limited plasticity of Th1 populations.
Figures


References
-
- Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. - PubMed
-
- Maloy KJ, Powrie F. Regulatory T cells in the control of immune pathology. Nat. Immunol. 2001;2:816–822. - PubMed
-
- Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat. Rev. Immunol. 2002;2:389–400. - PubMed
-
- Grogan JL, Mohrs M, Harmon B, Lacy DA, Sedat JW, Locksley RM. Early transcription and silencing of cytokine genes underlie polarization of T helper cell subsets. Immunity. 2001;14:205–215. - PubMed
-
- Mullen AC, Hutchins AS, Villarino AV, Lee HW, High FA, Cereb N, Yang SY, Hua X, Reiner SL. Cell cycle controlling the silencing and functioning of mammalian activators. Curr. Biol. 2001;11:1695–1699. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

