Direct CD1d-mediated stimulation of APC IL-12 production and protective immune response to virus infection in vivo
- PMID: 19949077
- PMCID: PMC2967442
- DOI: 10.4049/jimmunol.0800924
Direct CD1d-mediated stimulation of APC IL-12 production and protective immune response to virus infection in vivo
Abstract
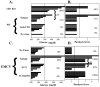
CD1d-restricted NKT cells rapidly stimulate innate and adaptive immunity through production of Th1 and/or Th2 cytokines and induction of CD1d(+) APC maturation. However, therapeutic exploitation of NKT cells has been hampered by their paucity and defects in human disease. NKT cell-APC interactions can be modeled by direct stimulation of human APCs through CD1d in vitro. We have now found that direct ligation with multiple CD1d mAbs also stimulated bioactive IL-12 release from CD1d(+) but not CD1d knockout murine splenocytes in vitro. Moreover, all of the CD1d mAbs tested also induced IL-12 as well as both IFN-gamma and IFN-alpha in vivo from CD1d(+) but not CD1d-deficient recipients. Unlike IFN-gamma, CD1d-induced IFN-alpha was at least partially dependent on invariant NKT cells. Optimal resistance to infection with picornavirus encephalomyocarditis virus is known to require CD1d-dependent APC IL-12-induced IFN-gamma as well as IFN-alpha. CD1d ligation in vivo enhanced systemic IL-12, IFN-gamma, and IFN-alpha and was protective against infection by encephalomyocarditis virus, suggesting an alternative interpretation for previous results involving CD1d "blocking" in other systems. Such protective responses, including elevations in Th1 cytokines, were also seen with CD1d F(ab')(2)s in vivo, whereas an IgM mAb (with presumably minimal tissue penetration) was comparably effective at protection in vivo as well as cytokine induction both in vivo and in vitro. Although presumably acting immediately "downstream," CD1d mAbs were protective later during infection than the invariant NKT cell agonist alpha-galactosylceramide. These data indicate that NKT cells can be bypassed with CD1d-mediated induction of robust Th1 immunity, which may have therapeutic potential both directly and as an adjuvant.
Figures





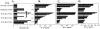
References
-
- Van Kaer L, Joyce S. Innate immunity: NKT cells in the spotlight. Curr. Biol. 2005;15:R429–431. - PubMed
-
- Kronenberg M, Rudensky A. Regulation of immunity by self-reactive T cells. Nature. 2005;435:598–604. - PubMed
-
- Bendelac A, Savage P, Teyton L. The biology of NKT cells. Annu. Rev. Immunol. 2007;25:297–336. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

