ATP-binding cassette transporter G1 negatively regulates thymocyte and peripheral lymphocyte proliferation
- PMID: 19949102
- PMCID: PMC3316475
- DOI: 10.4049/jimmunol.0902372
ATP-binding cassette transporter G1 negatively regulates thymocyte and peripheral lymphocyte proliferation
Abstract
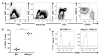
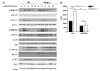
Cholesterol is a key component of cell membranes and is essential for cell growth and proliferation. How the accumulation of cellular cholesterol affects lymphocyte development and function is not well understood. We demonstrate that ATP-binding cassette transporter G1 (ABCG1) regulates cholesterol homeostasis in thymocytes and peripheral CD4 T cells. Our work is the first to describe a cell type in Abcg1-deficient mice with such a robust change in cholesterol content and the expression of cholesterol metabolism genes. Abcg1-deficient mice display increased thymocyte cellularity and enhanced proliferation of thymocytes and peripheral T lymphocytes in vivo. The absence of ABCG1 in CD4 T cells results in hyperproliferation in vitro, but only when cells are stimulated through the TCR. We hypothesize that cholesterol accumulation in Abcg1(-/-) T cells alters the plasma membrane structure, resulting in enhanced TCR signaling for proliferation. Supporting this idea, we demonstrate that B6 T cells pretreated with soluble cholesterol have a significant increase in proliferation. Cholesterol accumulation in Abcg1(-/-) CD4 T cells results in enhanced basal phosphorylation levels of ZAP70 and ERK1/2. Furthermore, inhibition of ERK phosphorylation in TCR-stimulated Abcg1(-/-) T cells rescues the hyperproliferative phenotype. We describe a novel mechanism by which cholesterol can alter signaling from the plasma membrane to affect downstream signaling pathways and proliferation. These results implicate ABCG1 as an important negative regulator of lymphocyte proliferation through the maintenance of cellular cholesterol homeostasis.
Figures





References
-
- Cantrell DA. Transgenic analysis of thymocyte signal transduction. Nat. Rev. Immunol. 2002;2:20–27. - PubMed
-
- Michie AM, Zúñiga-Pflücker JC. Regulation of thymocyte differentiation: pre-TCR signals and β-selection. Semin. Immunol. 2002;14:311–323. - PubMed
-
- Goldrath AW, Bevan MJ. Selecting and maintaining a diverse T-cell repertoire. Nature. 1999;402:255–262. - PubMed
-
- Freitas AA, Rocha B. Peripheral T cell survival. Curr. Opin. Immunol. 1999;11:152–156. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

