Differentiation stage-specific requirement in hypoxia-inducible factor-1alpha-regulated glycolytic pathway during murine B cell development in bone marrow
- PMID: 19949104
- PMCID: PMC2848717
- DOI: 10.4049/jimmunol.0800167
Differentiation stage-specific requirement in hypoxia-inducible factor-1alpha-regulated glycolytic pathway during murine B cell development in bone marrow
Abstract
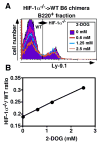
Hypoxia-inducible factor (HIF)-1alpha plays a central role in oxygen homeostasis and energy supply by glycolysis in many cell types. We previously reported that an HIF-1alpha gene deficiency caused abnormal B cell development and autoimmunity. In this study we show that HIF-1alpha-enabled glycolysis during B cell development is required in a developmental stage-specific manner. Supporting this conclusion are observations that the glycolytic pathway in HIF-1alpha-deficient B220(+) bone marrow cells is much less functionally effective than in wild-type control cells. The expression of genes encoding the glucose transporters and the key glycolytic enzyme, 6-phosphofructo-2-kinase/fructose-2,6-bishosphatase 3, was greatly reduced in HIF-1alpha-deficient cells. The compensatory adaptation to the defect of glycolysis was reflected in higher levels of expression of respiratory chain-related genes and TCA cycle-related genes in HIF-1alpha-deficient cells than in wild-type cells. In agreement with these findings, HIF-1alpha-deficient cells used pyruvate more efficiently than wild-type cells. The key role of HIF-1alpha-enabled glycolysis in bone marrow B cells was also demonstrated by glucose deprivation during in vitro bone marrow cell culture and by using a glycolysis inhibitor in the bone marrow cell culture. Taken together, these findings indicate that glucose dependency differs at different B cell developmental stages and that HIF-1alpha plays an important role in B cell development.
Conflict of interest statement
The authors have no financial conflict of interest.
Figures








References
-
- Caldwell CC, Kojima H, Lukashev D, Armstrong J, Farber M, Apasov SG, Sitkovsky MV. Differential effects of physiologically relevant hypoxic conditions on T lymphocyte development and effector functions. J Immunol. 2001;167:6140–6149. - PubMed
-
- Huang JH, Cardenas-Navia LI, Caldwell CC, Plumb TJ, Radu CG, Rocha PN, Wilder T, Bromberg JS, Cronstein BN, Sitkovsky M, Dewhirst MW, Dustin ML. Requirements for T lymphocyte migration in explanted lymph nodes. J Immunol. 2007;178:7747–7755. - PubMed
-
- Asosingh K, De Raeve H, de Ridder M, Storme GA, Willems A, Van Riet I, Van Camp B, Vanderkerken K. Role of the hypoxic bone marrow microenvironment in 5T2MM murine myeloma tumor progression. Haematology. 2005;90:810–817. - PubMed
-
- Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol. 1999;15:551–578. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

