Voltage-gated potassium channels as therapeutic targets
- PMID: 19949402
- PMCID: PMC2790170
- DOI: 10.1038/nrd2983
Voltage-gated potassium channels as therapeutic targets
Abstract
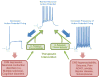
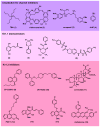
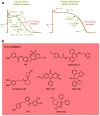
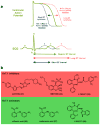
The human genome encodes 40 voltage-gated K(+) channels (K(V)), which are involved in diverse physiological processes ranging from repolarization of neuronal and cardiac action potentials, to regulating Ca(2+) signalling and cell volume, to driving cellular proliferation and migration. K(V) channels offer tremendous opportunities for the development of new drugs to treat cancer, autoimmune diseases and metabolic, neurological and cardiovascular disorders. This Review discusses pharmacological strategies for targeting K(V) channels with venom peptides, antibodies and small molecules, and highlights recent progress in the preclinical and clinical development of drugs targeting the K(V)1 subfamily, the K(V)7 subfamily (also known as KCNQ), K(V)10.1 (also known as EAG1 and KCNH1) and K(V)11.1 (also known as HERG and KCNH2) channels.
Figures


References
-
- Papazian DM, Schwarz TL, Tempel BL, Jan YN, Jan LY. Cloning of genomic and complementary DNA from Shaker, a putative potassium channel gene from Drosophila. Science. 1987;237:749–753. Study describing the cloning of the first KVchannel. - PubMed
-
- Long SB, Campbell EB, Mackinnon R. Crystal structure of a mammalian voltage-dependent Shaker family K+channel. Science. 2005;309:897–903. First description of the crystal structure of a voltage-gated mammalian K+ channel with its associated β-subunit. - PubMed
-
- Long SB, Campbell EB, Mackinnon R. Voltage sensor of Kv1.2: structural basis of electromechanical coupling. Science. 2005;309:903–908. Companion paper to Ref. 3 describing the structural basis of voltage-sensor and pore-domain coupling. - PubMed
-
- Swartz KJ. Towards a structural view of gating in potassium channels. Nat Rev Neurosci. 2004;5:905–916. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

