Surfactant therapy for neonatal respiratory distress syndrome: a review of Korean experiences over 17 years
- PMID: 19949668
- PMCID: PMC2775860
- DOI: 10.3346/jkms.2009.24.6.1110
Surfactant therapy for neonatal respiratory distress syndrome: a review of Korean experiences over 17 years
Abstract
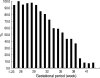
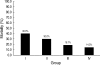
We undertook a multi-hospital collective study to evaluate outcomes of neonatal respiratory distress syndrome (RDS) patients treated with pulmonary surfactant (PS) over 17 yr in Korea (Group I; 1990/91, Group II; 1996, Group III; 2002, and Group IV; 2007). There were 60 neonates in Group I (16 hospitals), 1,179 in Group II (64), 1,595 in Group III (62), and 1,921 in Group IV (57). We adopted Bomsel's classification to evaluate initial chest radiographic findings, categorized RDS severities, and classified response types to PS therapy. Almost all cases were treated using a single dose in Groups I and II, but 19.5% received multiple-dose therapy in Group IV. In Group IV, Bomsel's stages III and IV composed 62.9% and initial severities of mild, moderate, and severe RDS were 23.0%, 42.0%, and 35.0%. More infants showed good response in Groups II, III, and IV than in Group I (71.7%, 66.8%, and 69.2% vs. 58.3%). Complications and mortality rate were lower in Group IV than in Groups I, II, and III (mortality rate: 14.3% vs. 40.0%, 30.0%, and 18.7%). We conclude that PS therapy in neonates with RDS had a remarkable impact on improving clinical course and outcomes over 17 yr in Korea.
Keywords: Analysis; Complications; Data Collection; Epidemiology; Mortality; Premature; Pulmonary Surfactants; Respiratory Distress Syndrome, Newborn; Therapeutic Use.
Figures
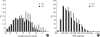

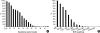





Similar articles
-
Pulmonary Surfactant Replacement Therapy for Respiratory Distress Syndrome in Neonates: a Nationwide Epidemiological Study in Korea.J Korean Med Sci. 2020 Aug 17;35(32):e253. doi: 10.3346/jkms.2020.35.e253. J Korean Med Sci. 2020. PMID: 32808509 Free PMC article.
-
[Effect of natural surfactant on mortality and morbidity in neonates with a birth weight below 1500 grams].Ceska Gynekol. 2000 Dec;65 Suppl 1:38-42. Ceska Gynekol. 2000. PMID: 11394231 Czech.
-
[Epidemiological survey of neonatal respiratory distress syndrome in part of northwest regions in China].Zhonghua Er Ke Za Zhi. 2015 May;53(5):341-7. Zhonghua Er Ke Za Zhi. 2015. PMID: 26080663 Chinese.
-
History of Pulmonary Surfactant Replacement Therapy for Neonatal Respiratory Distress Syndrome in Korea.J Korean Med Sci. 2019 Jul 1;34(25):e175. doi: 10.3346/jkms.2019.34.e175. J Korean Med Sci. 2019. PMID: 31243934 Free PMC article. Review.
-
Chronic lung disease of the very low birth weight infant--is it preventable?Turk J Pediatr. 1998 Jan-Mar;40(1):35-44. Turk J Pediatr. 1998. PMID: 9673527 Review.
Cited by
-
Permissive hyperglycemia in extremely low birth weight infants.J Korean Med Sci. 2013 Mar;28(3):450-60. doi: 10.3346/jkms.2013.28.3.450. Epub 2013 Mar 4. J Korean Med Sci. 2013. PMID: 23487562 Free PMC article.
-
Risk factors and neonatal outcomes of pulmonary air leak syndrome in extremely preterm infants: A nationwide descriptive cohort study.Medicine (Baltimore). 2023 Aug 25;102(34):e34759. doi: 10.1097/MD.0000000000034759. Medicine (Baltimore). 2023. PMID: 37653823 Free PMC article.
-
Prognosis of very preterm infants with severe respiratory distress syndrome receiving mechanical ventilation.Lung. 2015 Apr;193(2):249-54. doi: 10.1007/s00408-014-9683-5. Epub 2015 Jan 13. Lung. 2015. PMID: 25583617
-
Early prophylactic versus late selective use of surfactant for respiratory distress syndrome in very preterm infants: a collaborative study of 53 multi-center trials in Korea.J Korean Med Sci. 2014 Aug;29(8):1126-31. doi: 10.3346/jkms.2014.29.8.1126. Epub 2014 Jul 30. J Korean Med Sci. 2014. PMID: 25120324 Free PMC article. Clinical Trial.
-
Development of a Synthetic Surfactant Using a Surfactant Protein-C Peptide Analog: In Vitro Studies of Surface Physical Properties.Yonsei Med J. 2016 Jan;57(1):203-8. doi: 10.3349/ymj.2016.57.1.203. Yonsei Med J. 2016. PMID: 26632402 Free PMC article.
References
-
- Avery ME, Mead J. Surface properties in relation to atelectasis and hyaline membrane disease. AMA J Dis Child. 1959;97:517–523. - PubMed
-
- Fujiwara T, Maeta H, Chida S, Morita T, Watabe Y, Abe T. Artificial surfactant therapy in hyaline-membrane disease. Lancet. 1980;1:55–59. - PubMed
-
- Namgung R, Lee C, Park KI, Han DG. Exogenous surfactant replacement therapy of hyaline membrane disease: a controlled clinical trial. J Korean Pediatr Soc. 1990;33:22–35.
-
- Park CO, Lim BY, Yeo BG, Song JH, Sohn EK, Bae CW, Chung SJ, Ahn CI. Surfactant replacement therapy in neonatal respiratory distress syndrome. J Korean Pediatr Soc. 1991;34:1211–1222.
-
- Bae CW, kwon YD, Ko SJ, Kim KS, Kim HM, Park WS, Byun SH, Son CS, Ahn HS, Lee SG, Chang YP, Chung YJ. Surfactant replacement therapy in neonates with respiratory distress syndrome: a collective evaluation of trials from 16 hospitals. J Korean Pediatr Soc. 1993;36:244–265.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

