Preparation of intravenous stealthy acyclovir nanoparticles with increased mean residence time
- PMID: 19949904
- PMCID: PMC2799608
- DOI: 10.1208/s12249-009-9342-y
Preparation of intravenous stealthy acyclovir nanoparticles with increased mean residence time
Abstract
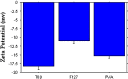
A major cause of thromboplebitis, during acyclovir (ACV) parenteral administration is the high pH of its reconstituted solution (pH 11). Its plasma half life is 2.5 h, requiring repeated administration which may result in excess of drug solubility leading to possible renal damage and acute renal failure. The present study reports the efficiency of stealthy ACV nanoparticles (NPs) to increase the mean residence time of the drug 29 times. It caused a marked decrease in thrombophlebitis when injected into rabbit's ear vein. The polymers used were (Poly lactic acid, polylactic-co-glycolic (PLGA) 85/15, PLGA 75/25, PLGA 50/50). Particles were evaluated for their encapsulation efficiency, morphology, particle size and size distribution, zeta potential, and in vitro drug release. Small NPs (280-300 nm) with 60% drug release after 48 h were obtained. Among the block copolymer used, poloxamer 407 was of superior coating properties with a coat thickness in the range of 1.5-8.3 nm and a decreased surface charge.
Figures






Similar articles
-
Ocular sustained release nanoparticles containing stereoisomeric dipeptide prodrugs of acyclovir.J Ocul Pharmacol Ther. 2011 Apr;27(2):163-72. doi: 10.1089/jop.2010.0188. J Ocul Pharmacol Ther. 2011. PMID: 21500985 Free PMC article.
-
Galactose decorated PLGA nanoparticles for hepatic delivery of acyclovir.Drug Dev Ind Pharm. 2013 Dec;39(12):1866-73. doi: 10.3109/03639045.2012.662510. Epub 2012 Mar 8. Drug Dev Ind Pharm. 2013. PMID: 22397550
-
Topical application of acyclovir-loaded microparticles: quantification of the drug in porcine skin layers.J Control Release. 2001 Jul 10;75(1-2):191-7. doi: 10.1016/s0168-3659(01)00395-9. J Control Release. 2001. PMID: 11451509
-
PEGylated Lipid Polymeric Nanoparticle-Encapsulated Acyclovir for In Vitro Controlled Release and Ex Vivo Gut Sac Permeation.AAPS PharmSciTech. 2020 Oct 14;21(7):285. doi: 10.1208/s12249-020-01810-0. AAPS PharmSciTech. 2020. PMID: 33057878 Free PMC article.
-
Nano and microparticulate chitosan-based systems for antiviral topical delivery.Eur J Pharm Sci. 2013 Jan 23;48(1-2):216-22. doi: 10.1016/j.ejps.2012.11.002. Epub 2012 Nov 16. Eur J Pharm Sci. 2013. PMID: 23159663
Cited by
-
Critical evaluation of biodegradable polymers used in nanodrugs.Int J Nanomedicine. 2013;8:3071-90. doi: 10.2147/IJN.S47186. Epub 2013 Aug 19. Int J Nanomedicine. 2013. PMID: 23990720 Free PMC article. Review.
-
Niosomes: A Strategy toward Prevention of Clinically Significant Drug Incompatibilities.Sci Rep. 2017 Jul 24;7(1):6340. doi: 10.1038/s41598-017-06955-w. Sci Rep. 2017. PMID: 28740102 Free PMC article.
-
Cubogel as potential platform for glaucoma management.Drug Deliv. 2021 Dec;28(1):293-305. doi: 10.1080/10717544.2021.1872740. Drug Deliv. 2021. PMID: 33509004 Free PMC article.
-
Nano Drug Delivery Platforms for Dental Application: Infection Control and TMJ Management-A Review.Polymers (Basel). 2021 Nov 29;13(23):4175. doi: 10.3390/polym13234175. Polymers (Basel). 2021. PMID: 34883678 Free PMC article.
-
Polymer-Based Nanoparticles as Drug Delivery Systems for Purines of Established Importance in Medicine.Nanomaterials (Basel). 2023 Sep 26;13(19):2647. doi: 10.3390/nano13192647. Nanomaterials (Basel). 2023. PMID: 37836288 Free PMC article. Review.
References
-
- Johnson RJ. The complement system. In: Ratner BD, Hoffman AS, Schoen FJ, Lemons JE, editors. Biomaterials science: an introduction to materials in medicine. Amesterdam: Elsevier Academic Press; 2004. pp. 318–328.
-
- de Miranda P, Blum MR. Pharmacokinetics of Acyclovir after intravenous and oral administration. J Antimicrob Chemother. 1983;12(Suppl. B):29–37. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

