The inhibition of pyruvate dehydrogenase kinase improves impaired cardiac function and electrical remodeling in two models of right ventricular hypertrophy: resuscitating the hibernating right ventricle
- PMID: 19949938
- PMCID: PMC3155251
- DOI: 10.1007/s00109-009-0524-6
The inhibition of pyruvate dehydrogenase kinase improves impaired cardiac function and electrical remodeling in two models of right ventricular hypertrophy: resuscitating the hibernating right ventricle
Abstract
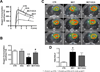
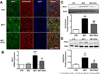
Right ventricular hypertrophy (RVH) and RV failure contribute to morbidity and mortality in pulmonary arterial hypertension (PAH). The cause of RV dysfunction and the feasibility of therapeutically targeting the RV are uncertain. We hypothesized that RV dysfunction and electrical remodeling in RVH result, in part, from a glycolytic shift in the myocyte, caused by activation of pyruvate dehydrogenase kinase (PDK). We studied two complementary rat models: RVH + PAH (induced by monocrotaline) and RVH + without PAH (induced by pulmonary artery banding (PAB)). Monocrotaline RVH reduced RV O(2)-consumption and enhanced glycolysis. RV 2-fluoro-2-deoxy-glucose uptake, Glut-1 expression, and pyruvate dehydrogenase phosphorylation increased in monocrotaline RVH. The RV monophasic action potential duration and QT(c) interval were prolonged due to decreased expression of repolarizing voltage-gated K(+) channels (Kv1.5, Kv4.2). In the RV working heart model, the PDK inhibitor, dichloroacetate, acutely increased glucose oxidation and cardiac work in monocrotaline RVH. Chronic dichloroacetate therapy improved RV repolarization and RV function in vivo and in the RV Langendorff model. In PAB-induced RVH, a similar reduction in cardiac output and glycolytic shift occurred and it too improved with dichloroacetate. In PAB-RVH, the benefit of dichloroacetate on cardiac output was approximately 1/3 that in monocrotaline RVH. The larger effects in monocrotaline RVH likely reflect dichloroacetate's dual metabolic benefits in that model: regression of vascular disease and direct effects on the RV. Reduction in RV function and electrical remodeling in two models of RVH relevant to human disease (PAH and pulmonic stenosis) result, in part, from a PDK-mediated glycolytic shift in the RV. PDK inhibition partially restores RV function and regresses RVH by restoring RV repolarization and enhancing glucose oxidation. Recognition that a PDK-mediated metabolic shift contributes to contractile and ionic dysfunction in RVH offers insight into the pathophysiology and treatment of RVH.
Conflict of interest statement
The authors have no conflicts to disclose.
Figures

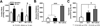




References
-
- Bonnet S, Michelakis ED, Porter CJ, Andrade-Navarro MA, Thebaud B, Haromy A, Harry G, Moudgil R, McMurtry MS, Weir EK, Archer SL. An abnormal mitochondrial-hypoxia inducible factor-1alpha-Kv channel pathway disrupts oxygen sensing and triggers pulmonary arterial hypertension in fawn hooded rats: similarities to human pulmonary arterial hypertension. Circulation. 2006;113:2630–2641. - PubMed
-
- van Wolferen SA, Marcus JT, Boonstra A, Marques KM, Bronzwaer JG, Spreeuwenberg MD, Postmus PE, Vonk-Noordegraaf A. Prognostic value of right ventricular mass, volume, and function in idiopathic pulmonary arterial hypertension. Eur Heart J. 2007;28:1250–1257. - PubMed
-
- Hessel MH, Steendijk P, den Adel B, Schutte CI, van der Laarse A. Characterization of right ventricular function after monocrotaline-induced pulmonary hypertension in the intact rat. Am J Physiol Heart Circ Physiol. 2006;291:H2424–H2430. - PubMed
-
- Lamberts RR, Caldenhoven E, Lansink M, Witte G, Vaessen RJ, St Cyr JA, Stienen GJ. Preservation of diastolic function in monocrotaline-induced right ventricular hypertrophy in rats. Am J Physiol Heart Circ Physiol. 2007;293:H1869–H1876. - PubMed
-
- Lee JK, Kodama I, Honjo H, Anno T, Kamiya K, Toyama J. Stage-dependent changes in membrane currents in rats with monocrotaline-induced right ventricular hypertrophy. Am J Physiol. 1997;272:H2833–H2842. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

