Molecular imaging of the translocator protein (TSPO) in a pre-clinical model of breast cancer
- PMID: 19949989
- PMCID: PMC6544016
- DOI: 10.1007/s11307-009-0270-8
Molecular imaging of the translocator protein (TSPO) in a pre-clinical model of breast cancer
Abstract
Purpose: To quantitatively evaluate the utility of a translocator protein (TSPO)-targeted near-infrared (NIR) probe (NIR-conPK11195) for in vivo molecular imaging of TSPO in breast cancer.
Procedures: NIR-conPK11195 uptake and TSPO-specificity were validated in TSPO-expressing human breast adenocarcinoma cells (MDA-MB-231). In vivo NIR-conPK11195 biodistribution and accumulation were quantitatively evaluated in athymic nude mice bearing MDA-MB-231 xenografts.
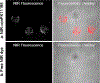
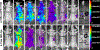
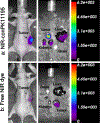
Results: Fluorescence micrographs illustrated intracellular labeling of MDA-MB-231 cells by NIR-conPK11195. Quantitative uptake and competition assays demonstrated dose-dependent (p < 0.001) and TSPO-specific (p < 0.001) NIR-conPK11195 uptake. In vivo, NIR-conPK11195 preferentially labeled MDA-MB-231 tumors with an 11-fold (p < 0.001) and 7-fold (p < 0.001) contrast enhancement over normal tissue and unconjugated NIR dye, respectively.
Conclusions: NIR-conPK11195 appears to be a promising TSPO-targeted molecular imaging agent for visualization and quantification of breast cancer cells in vivo. This research represents the first study to demonstrate the feasibility of TSPO imaging as an alternative breast cancer imaging approach.
Figures
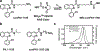


References
-
- American Cancer Society (2009) Cancer Facts and Figures Atlanta: American Cancer Society
-
- Carney PA, Miglioretti DL, Yankaskas BC et al. (2003) Individual and combined effects of age, breast density, and hormone replacement therapy use on the accuracy of screening mammography. Ann Intern Med 138(3):168–175 - PubMed
-
- DeMartini W, Lehman C, Partridge S (2008) Breast MRI for cancer detection and characterization: a review of evidence-based clinical applications. Acad Radiol 15(4):408–416 - PubMed
-
- Stavros AT, Thickman D, Rapp CL, Dennis MA, Parker SH, Sisney GA (1995) Solid breast nodules: use of sonography to distinguish between benign and malignant lesions. Radiology 196(1):123–134 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

