Plasma cell toll-like receptor (TLR) expression differs from that of B cells, and plasma cell TLR triggering enhances immunoglobulin production
- PMID: 19950420
- PMCID: PMC2792141
- DOI: 10.1111/j.1365-2567.2009.03143.x
Plasma cell toll-like receptor (TLR) expression differs from that of B cells, and plasma cell TLR triggering enhances immunoglobulin production
Abstract
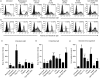
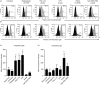
Toll-like receptors (TLRs) are key receptors of the innate immune system and show cell subset-specific expression. We investigated the messenger RNA (mRNA) expression of TLR genes in human haematopoietic stem cells (HSC), in naïve B cells, in memory B cells, in plasma cells from palatine tonsils and in plasma cells from peripheral blood. HSC and plasma cells showed unrestricted expression of TLR1-TLR9, in contrast to B cells which lacked TLR3, TLR4 and TLR8 but expressed mRNA of all other TLRs. We demonstrated, for the first time, that TLR triggering of terminally differentiated plasma cells augments immunoglobulin production. Thus, boosting the immediate antibody response by plasma cells upon pathogen recognition may point to a novel role of TLRs.
Figures

Similar articles
-
Toll-like receptor agonists synergize with CD40L to induce either proliferation or plasma cell differentiation of mouse B cells.PLoS One. 2011;6(10):e25542. doi: 10.1371/journal.pone.0025542. Epub 2011 Oct 3. PLoS One. 2011. PMID: 21991317 Free PMC article.
-
Suppression of B-cell activation and IgE, IgA, IgG1 and IgG4 production by mammalian telomeric oligonucleotides.Allergy. 2013;68(5):593-603. doi: 10.1111/all.12133. Epub 2013 Mar 9. Allergy. 2013. PMID: 23480796
-
Toll-like receptors in human chondrocytes and osteoarthritic cartilage.Acta Orthop. 2013 Dec;84(6):585-92. doi: 10.3109/17453674.2013.854666. Epub 2013 Nov 18. Acta Orthop. 2013. PMID: 24237425 Free PMC article.
-
Expression and function of Toll-like receptors in T lymphocytes.Curr Opin Immunol. 2007 Feb;19(1):39-45. doi: 10.1016/j.coi.2006.11.007. Epub 2006 Nov 28. Curr Opin Immunol. 2007. PMID: 17129718 Review.
-
Toll-like receptors in bony fish: from genomics to function.Dev Comp Immunol. 2011 Dec;35(12):1263-72. doi: 10.1016/j.dci.2011.03.006. Epub 2011 Mar 15. Dev Comp Immunol. 2011. PMID: 21414346 Review.
Cited by
-
Expression and functionality of Toll-like receptor 3 in the megakaryocytic lineage.J Thromb Haemost. 2015 May;13(5):839-50. doi: 10.1111/jth.12842. Epub 2015 Feb 20. J Thromb Haemost. 2015. PMID: 25594115 Free PMC article.
-
Toll-Like Receptor 4 Activation Promotes Multiple Myeloma Cell Growth and Survival Via Suppression of The Endoplasmic Reticulum Stress Factor Chop.Sci Rep. 2019 Mar 1;9(1):3245. doi: 10.1038/s41598-019-39672-7. Sci Rep. 2019. PMID: 30824741 Free PMC article.
-
CD27hiCD38hi plasmablasts are activated B cells of mixed origin with distinct function.iScience. 2021 Apr 29;24(5):102482. doi: 10.1016/j.isci.2021.102482. eCollection 2021 May 21. iScience. 2021. PMID: 34113823 Free PMC article.
-
Innate immune responses against Epstein Barr virus infection.J Leukoc Biol. 2013 Dec;94(6):1185-90. doi: 10.1189/jlb.0313173. Epub 2013 Jun 28. J Leukoc Biol. 2013. PMID: 23812328 Free PMC article. Review.
-
Toll-Like Receptor Signaling and Immune Regulatory Lymphocytes in Periodontal Disease.Int J Mol Sci. 2020 May 8;21(9):3329. doi: 10.3390/ijms21093329. Int J Mol Sci. 2020. PMID: 32397173 Free PMC article. Review.
References
-
- Ishii KJ, Koyama S, Nakagawa A, Coban C, Akira S. Host innate immune receptors and beyond: making sense of microbial infections. Cell Host Microbe. 2008;3:352–63. - PubMed
-
- Hayashi F, Means TK, Luster AD. Toll-like receptors stimulate human neutrophil function. Blood. 2003;102:2660–9. - PubMed
-
- Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdorfer B, Giese T, Endres S, Hartmann G. Quantitative expression of toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol. 2002;168:4531–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

