Improved virological outcomes in British Columbia concomitant with decreasing incidence of HIV type 1 drug resistance detection
- PMID: 19951169
- PMCID: PMC2922860
- DOI: 10.1086/648729
Improved virological outcomes in British Columbia concomitant with decreasing incidence of HIV type 1 drug resistance detection
Abstract
Background: There have been limited studies evaluating temporal changes in the incidence of detection of drug resistance among human immunodeficiency virus type 1 (HIV-1) isolates and concomitant changes in plasma HIV load for treated individuals in a population-wide setting.
Methods: Longitudinal plasma viral load and genotypic resistance data were obtained from patients receiving antiretroviral therapy from the British Columbia Drug Treatment Program from July 1996 through December 2008. A total of 24,652 resistance tests were available from 5422 individuals. The incidence of successful plasma viral load suppression and of resistance to each of 3 antiretroviral categories (nucleoside/nucleotide reverse-transcriptase inhibitors, nonnucleoside reverse transcriptase inhibitors, and protease inhibitors) was calculated for the population receiving therapy.
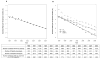
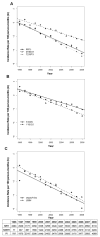
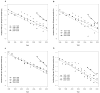
Results: There has been a drastic decrease in the incidence of new cases of HIV-1 drug resistance in individuals followed during 1996-2008. In 1997, the incidence rate of any newly detected resistance was 1.73 cases per 100 person-months of therapy, and by 2008, the incidence rate had decreased >12-fold, to 0.13 cases per 100 person-months of therapy. This decrease in the incidence of resistance has occurred at an exponential rate, with half-times on the order of 2-3 years. Concomitantly, the proportion of individuals with plasma viral load suppression has increased linearly over time (from 64.7% with HIV RNA levels <50 copies/mL in 2000 to 87.0% in 2008; R2=0.97; P<.001).
Conclusions: Our results suggest an increasing effectiveness of highly active antiretroviral therapy at the populational level. The vast majority of treated patients in British Columbia now have either suppressed plasma viral load or drug-susceptible HIV-1, according to their most recent test results.
Conflict of interest statement
Figures

References
-
- Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–60. - PubMed
-
- Panos G, Charatsis G, Paparizos V, Kazantzi M, Falagas ME. Prevalence of genotypic resistance to nucleoside analogues, nonnucleoside analogues, and protease inhibitors in HIV-infected persons in Athens, Greece. AIDS Res Hum Retroviruses. 2008;24:43–51. - PubMed
-
- Escoto-Delgadillo M, Vazquez-Valls E, Ramirez-Rodriguez M, et al. Drug-resistance mutations in antiretroviral-naive patients with established HIV-1 infection in Mexico. HIV Med. 2005;6:403–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

