Mirasol Pathogen Reduction Technology treatment does not affect acute lung injury in a two-event in vivo model caused by stored blood components
- PMID: 19951305
- PMCID: PMC3132808
- DOI: 10.1111/j.1423-0410.2009.01289.x
Mirasol Pathogen Reduction Technology treatment does not affect acute lung injury in a two-event in vivo model caused by stored blood components
Abstract
Introduction: Mirasol Pathogen Reduction Technology (PRT) treatment uses riboflavin and UV light to inactivate pathogens in blood components. Neutrophil [polymorphonuclear cells (PMN)] priming activity accumulates during routine storage of cellular blood components, and this activity has been implicated in transfusion-related acute lung injury (TRALI). We hypothesize that PRT-treatment of blood components affects the priming activity generated during storage of packed RBCs (PRBCs) or platelet concentrates (PCs), which can elicit ALI in vivo.
Methods: Plasma, PRBCs and PCs were isolated from healthy donor's whole blood or by apheresis. Half of a collected unit was treated with PRT treatment and the remainder was left as an unmodified control. Supernatant was collected during storage of PCs and PRBCs and assayed for PMN priming activity and used as the second event in a two-event in vivo model of TRALI.
Results: PRT treatment did not induce priming activity in plasma or affect the priming activity generated during storage of PCs or PRBCs as compared with the unmodified controls. The supernatants from stored, but not fresh, PCs and PRBCs did cause ALI as the second event in a two-event animal model of TRALI, which was unaffected by PRT treatment. We conclude that the PRT treatment does not induce priming activity in plasma nor does it affect the priming activity generated during storage of PCs or PRBCs or their ability to cause ALI as the second event in a two-event in vivo model of TRALI. Moreover, the amount of priming activity in TRIMA-isolated PCs was significantly less than SPECTRA-isolated PCs.
Figures
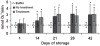
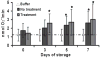
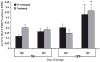

Similar articles
-
Human platelets pathogen reduced with riboflavin and ultraviolet light do not cause acute lung injury in a two-event SCID mouse model.Transfusion. 2014 Jan;54(1):74-85. doi: 10.1111/trf.12242. Epub 2013 May 9. Transfusion. 2014. PMID: 23656563
-
Generation of neutrophil priming activity by cell-containing blood components treated with pathogen reduction technology and stored in platelet additive solutions.Transfusion. 2011 Jun;51(6):1220-7. doi: 10.1111/j.1537-2995.2010.02983.x. Epub 2010 Dec 13. Transfusion. 2011. PMID: 21155831
-
Effects of a new pathogen-reduction technology (Mirasol PRT) on functional aspects of platelet concentrates.Transfusion. 2005 Jun;45(6):911-9. doi: 10.1111/j.1537-2995.2005.04350.x. Transfusion. 2005. PMID: 15934989
-
Hemovigilance survey of pathogen-reduced blood components in the Warsaw Region in the 2009 to 2013 period.Transfusion. 2016 Mar;56 Suppl 1:S39-44. doi: 10.1111/trf.13330. Transfusion. 2016. PMID: 27001360 Review.
-
The clinical and biological impact of new pathogen inactivation technologies on platelet concentrates.Blood Rev. 2014 Nov;28(6):235-41. doi: 10.1016/j.blre.2014.07.005. Epub 2014 Aug 24. Blood Rev. 2014. PMID: 25192602 Review.
Cited by
-
Real-Time Live Confocal Fluorescence Microscopy as a New Tool for Assessing Platelet Vitality.Transfus Med Hemother. 2010;37(5):299-305. doi: 10.1159/000320368. Epub 2010 Sep 15. Transfus Med Hemother. 2010. PMID: 21113254 Free PMC article.
-
Biological response modifiers in photochemically pathogen-reduced versus untreated apheresis platelet concentrates.Transfusion. 2013 Jan;53(1):147-55. doi: 10.1111/j.1537-2995.2012.03681.x. Epub 2012 May 7. Transfusion. 2013. PMID: 22563732 Free PMC article.
-
Age of blood and recipient factors determine the severity of transfusion-related acute lung injury (TRALI).Crit Care. 2012 Feb 1;16(1):R19. doi: 10.1186/cc11178. Crit Care. 2012. PMID: 22297161 Free PMC article.
-
Aged versus fresh autologous platelet transfusion in a two-hit healthy volunteer model of transfusion-related acute lung injury.Transfusion. 2022 Dec;62(12):2490-2501. doi: 10.1111/trf.17157. Epub 2022 Oct 27. Transfusion. 2022. PMID: 36300793 Free PMC article. Clinical Trial.
-
Update on transfusion-related acute lung injury: an overview of its pathogenesis and management.Front Immunol. 2023 May 12;14:1175387. doi: 10.3389/fimmu.2023.1175387. eCollection 2023. Front Immunol. 2023. PMID: 37251400 Free PMC article. Review.
References
-
- Solheim BG. Pathogen reduction of blood components. Transfus Apher Sci. 2008;39:75–82. - PubMed
-
- Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, LeGall JR, Morris A, Spragg R. Report of the American-European Consensus conference on acute respiratory distress syndrome: definitions, mechanisms, relevant outcomes, and clinical trial coordination. Consensus Committee. J Crit Care. 1994;9:72–81. - PubMed
-
- Kleinman S, Caulfield T, Chan P, Davenport R, McFarland J, McPhedran S, Meade M, Morrison D, Pinsent T, Robillard P, Slinger P. Toward an understanding of transfusion-related acute lung injury: statement of a consensus panel. Transfusion. 2004;44:1774–1789. - PubMed
-
- Toy P, Popovsky MA, Abraham E, Ambruso DR, Holness LG, Kopko PM, McFarland JG, Nathens AB, Silliman CC, Stroncek D. Transfusion-related acute lung injury: definition and review. Crit Care Med. 2005;33:721–726. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

