SKPs derive from hair follicle precursors and exhibit properties of adult dermal stem cells
- PMID: 19951689
- PMCID: PMC2828150
- DOI: 10.1016/j.stem.2009.10.019
SKPs derive from hair follicle precursors and exhibit properties of adult dermal stem cells
Abstract
Despite the remarkable regenerative capacity of mammalian skin, an adult dermal stem cell has not yet been identified. Here, we investigated whether skin-derived precursors (SKPs) might fulfill such a role. We show that SKPs derive from Sox2(+) hair follicle dermal cells and that these two cell populations are similar with regard to their transcriptome and functional properties. Both clonal SKPs and endogenous Sox2(+) cells induce hair morphogenesis, differentiate into dermal cell types, and home to a hair follicle niche upon transplantation. Moreover, hair follicle-derived SKPs self-renew, maintain their multipotency, and serially reconstitute hair follicles. Finally, grafting experiments show that follicle-associated dermal cells move out of their niche to contribute cells for dermal maintenance and wound-healing. Thus, SKPs derive from Sox2(+) follicle-associated dermal precursors and display functional properties predicted of a dermal stem cell, contributing to dermal maintenance, wound-healing, and hair follicle morphogenesis.
Figures
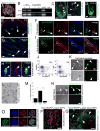
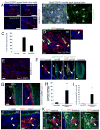

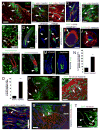

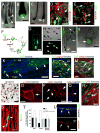

Comment in
-
Preview. SKPing a hurdle: Sox2 and adult dermal stem cells.Cell Stem Cell. 2009 Dec 4;5(6):569-70. doi: 10.1016/j.stem.2009.11.010. Cell Stem Cell. 2009. PMID: 19951681
References
-
- Biernaskie J, McKenzie IA, Toma JG, Miller FD. Isolation of skin-derived precursors (SKPs) and differentiation and enrichment of their Schwann cell progeny. Nat Protocols. 2006;1:2803–2812. - PubMed
-
- Ellis P, Fagan BM, Magness ST, Hutton S, Taranova O, Hayashi S, McMahon A, Rao M, Pevny L. SOX2, a persistent marker for multipotential neural stem cells derived from embryonic stem cells, the embryo or the adult. Dev Neurosci. 2004;26:148–165. - PubMed
-
- Fernandes KJ, Kobayashi NR, Gallagher CJ, Barnabe-Heider F, Aumont A, Kaplan DR, Miller FD. Analysis of the neurogenic potential of multipotent skin-derived precursors. Exp Neurol. 2006;201:32–48. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

