Tailoring iron chelation by iron intake and serum ferritin: the prospective EPIC study of deferasirox in 1744 patients with transfusion-dependent anemias
- PMID: 19951979
- PMCID: PMC2857545
- DOI: 10.3324/haematol.2009.014696
Tailoring iron chelation by iron intake and serum ferritin: the prospective EPIC study of deferasirox in 1744 patients with transfusion-dependent anemias
Abstract
Background Following a clinical evaluation of deferasirox (Exjade) it was concluded that, in addition to baseline body iron burden, ongoing transfusional iron intake should be considered when selecting doses. The 1-year EPIC study, the largest ever investigation conducted for an iron chelator, is the first to evaluate whether fixed starting doses of deferasirox, based on transfusional iron intake, with dose titration guided by serum ferritin trends and safety markers, provides clinically acceptable chelation in patients (aged >or=2 years) with transfusional hemosiderosis from various types of anemia.
Design and methods: The recommended initial dose was 20 mg/kg/day for patients receiving 2-4 packed red blood cell units/month and 10 or 30 mg/kg/day was recommended for patients receiving less or more frequent transfusions, respectively. Dose adjustments were based on 3-month serum ferritin trends and continuous assessment of safety markers. The primary efficacy end-point was change in serum ferritin after 52 weeks compared with baseline.
Results: The 1744 patients enrolled had the following conditions; thalassemia (n=1115), myelodysplastic syndromes (n=341), aplastic anemia (n=116), sickle cell disease (n=80), rare anemias (n=43) and other transfused anemias (n=49). Overall, there was a significant reduction in serum ferritin from baseline (-264 ng/mL; P<0.0001), reflecting dosage adjustments and ongoing iron intake. The most common (>5%) adverse events were gastrointestinal disturbances (28%) and skin rash (10%). Conclusions Analysis of this large, prospectively collected data set confirms the response to chelation therapy across various anemias, supporting initial deferasirox doses based on transfusional iron intake, with subsequent dose titration guided by trends in serum ferritin and safety markers (clinicaltrials.gov identifier: NCT00171821).
Figures
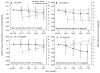
References
-
- Olivieri NF. The β-thalassemias. N Engl J Med. 1999;341(2):99–109. - PubMed
-
- Porter JB. Practical management of iron overload. Br J Haematol. 2001;115(2):239– 52. - PubMed
-
- Borgna-Pignatti C, Rugolotto S, De Stefano P, Zhao H, Cappellini MD, Del Vecchio GC, et al. Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica. 2004;89(10):1187–93. - PubMed
-
- Leitch HA. Improving clinical outcome in patients with myelodysplastic syndrome and iron overload using iron chelation therapy. Leuk Res. 2007;31 (Suppl 3):S7–9. - PubMed
-
- Rose C, Brechignac S, Vassilief D, Beyne-Rauzy O, Stamatoullas A, Larbaa D, et al. Positive impact of iron chelation therapy (CT) on survival in regularly transfused MDS patients. A prospective analysis by the GFM. Blood. 2007;110(11) abst 249.

