Femoral head deformation and repair following induction of ischemic necrosis: a histologic and magnetic resonance imaging study in the piglet
- PMID: 19952254
- PMCID: PMC2780921
- DOI: 10.2106/JBJS.H.01464
Femoral head deformation and repair following induction of ischemic necrosis: a histologic and magnetic resonance imaging study in the piglet
Abstract
Background: Ischemic necrosis of the femoral head can be induced surgically in the piglet. We used this model to assess femoral head deformation and repair in vivo by sequential magnetic resonance imaging and by correlating end-stage findings with histologic assessments.
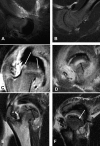
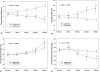
Methods: Ischemic necrosis of the femoral head was induced in ten three-week-old piglets by tying a silk ligature around the base of the femoral neck (intracapsular) and cutting the ligamentum teres. We used magnetic resonance imaging with the piglets under general anesthesia to study the hips at forty-eight hours and at one, two, four, and eight weeks. Measurements on magnetic resonance images in the midcoronal plane of the involved and control sides at each time documented the femoral head height, femoral head width, superior surface cartilage height, and femoral neck-shaft angle. Histologic assessments were done at the time of killing.
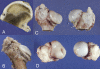
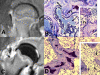
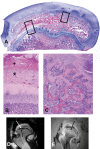
Results: Complete ischemia of the femoral head was identified in all involved femora by magnetic resonance imaging at forty-eight hours. Revascularization began at the periphery of the femoral head as early as one week and was underway in all by two weeks. At eight weeks, magnetic resonance imaging and histologic analysis showed deformation of the femoral head and variable tissue deposition. Tissue responses included (1) vascularized fibroblastic ingrowth with tissue resorption and cartilage, intramembranous bone, and mixed fibro-osseous or fibro-cartilaginous tissue synthesis and (2) resumption of endochondral bone growth. At eight weeks, the mean femoral head measurements (and standard error of the mean) for the control compared with the ligated femora were 10.4 +/- 0.4 and 4.8 +/- 0.4 mm, respectively, for height; 26.7 +/- 0.8 and 31.2 +/- 0.8 mm for diameter; 1.1 +/- 0.1 and 2.3 +/- 0.1 mm for cartilage thickness; and 151 degrees +/- 2 degrees and 135 degrees +/- 2 degrees for the femoral neck-shaft angle. Repeated-measures mixed-model analysis of variance revealed highly significant effects of ligation in each parameter (p < 0.0001).
Conclusions: Magnetic resonance imaging allows for the assessment of individual hips at sequential time periods to follow deformation and repair. There was a variable tissue response, and histologic assessment at the time of killing was shown to correlate with the evolving and varying magnetic resonance imaging signal intensities. Femoral head height on the ischemic side from one week onward was always less than the initial control value and continually decreased with time, indicating collapse as well as slowed growth. Increased femoral head width occurred relatively late (four to eight weeks), indicating cartilage model overgrowth concentrated at the periphery.
Figures

Similar articles
-
MRI of the cartilaginous epiphysis of the femoral head in the piglet hip after ischemic damage.J Magn Reson Imaging. 1998 May-Jun;8(3):717-23. doi: 10.1002/jmri.1880080331. J Magn Reson Imaging. 1998. PMID: 9626892
-
Ibandronate for prevention of femoral head deformity after ischemic necrosis of the capital femoral epiphysis in immature pigs.J Bone Joint Surg Am. 2005 Mar;87(3):550-7. doi: 10.2106/JBJS.D.02192. J Bone Joint Surg Am. 2005. PMID: 15741621
-
Subchondral fracture after ischemic osteonecrosis of the immature femoral head in piglet model.J Pediatr Orthop B. 2011 Jul;20(4):227-31. doi: 10.1097/BPB.0b013e328346725f. J Pediatr Orthop B. 2011. PMID: 21659956
-
Microcrack density and nanomechanical properties in the subchondral region of the immature piglet femoral head following ischemic osteonecrosis.Bone. 2013 Feb;52(2):632-9. doi: 10.1016/j.bone.2012.07.028. Epub 2012 Aug 3. Bone. 2013. PMID: 22889721
-
Osteonecrosis of the femoral head. Potential treatment with growth and differentiation factors.Clin Orthop Relat Res. 1998 Oct;(355 Suppl):S314-35. Clin Orthop Relat Res. 1998. PMID: 9917651 Review.
Cited by
-
Blood supply to the chicken femoral head.Comp Med. 2010 Aug;60(4):295-9. Comp Med. 2010. PMID: 20819379 Free PMC article.
-
A novel model of traumatic femoral head necrosis in rats developed by microsurgical technique.BMC Musculoskelet Disord. 2022 Apr 21;23(1):374. doi: 10.1186/s12891-022-05289-7. BMC Musculoskelet Disord. 2022. PMID: 35449009 Free PMC article.
-
Advances in experimental models of osteonecrosis of the femoral head.J Orthop Translat. 2023 Feb 3;39:88-99. doi: 10.1016/j.jot.2023.01.003. eCollection 2023 Mar. J Orthop Translat. 2023. PMID: 36819298 Free PMC article. Review.
-
Animal Model Standardization for Studying Avascular Necrosis of the Femoral Head in Legg-Calvé-Perthes Disease.Rev Bras Ortop (Sao Paulo). 2022 Oct 13;58(5):e771-e780. doi: 10.1055/s-0042-1749418. eCollection 2023 Oct. Rev Bras Ortop (Sao Paulo). 2022. PMID: 37908528 Free PMC article.
-
PlA2 Polymorphism of Platelet Glycoprotein IIb/IIIa and C677T Polymorphism of Methylenetetrahydrofolate Reductase (MTHFR), but Not Factor V Leiden and Prothrombin G20210A Polymorphisms, Are Associated with More Severe Forms of Legg-Calvé-Perthes Disease.Children (Basel). 2021 Jul 20;8(7):614. doi: 10.3390/children8070614. Children (Basel). 2021. PMID: 34356593 Free PMC article.
References
-
- Kim HK, Su PH, Qiu YS. Histopathologic changes in growth-plate cartilage following ischemic necrosis of the capital femoral epiphysis. An experimental investigation in immature pigs. J Bone Joint Surg Am. 2001;83:688-97. - PubMed
-
- Kim HK, Su PH. Development of flattening and apparent fragmentation following ischemic necrosis of the capital femoral epiphysis in a piglet model. J Bone Joint Surg Am. 2002;84:1329-34. - PubMed
-
- Jaramillo D, Connolly SA, Vajapeyam S, Robertson RL, Dunning PS, Mulkern RV, Hayward A, Maier SE, Shapiro F. Normal and ischemic epiphysis of the femur: diffusion MR imaging study in piglets. Radiology. 2003;227:825-32. - PubMed
-
- Menezes NM, Connolly SA, Shapiro F, Olear EA, Jimenez RM, Zurakowski D, Jaramillo D. Early ischemia in growing piglet skeleton: MR diffusion and perfusion imaging. Radiology. 2007;242:129-36. - PubMed
-
- Pringle D, Koob TJ, Kim HK. Indentation properties of growing femoral head following ischemic necrosis. J Orthop Res. 2004;22:122-30. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

