Global metabolic consequences of the chromogranin A-null model of hypertension: transcriptomic detection, pathway identification, and experimental verification
- PMID: 19952279
- PMCID: PMC2825767
- DOI: 10.1152/physiolgenomics.00164.2009
Global metabolic consequences of the chromogranin A-null model of hypertension: transcriptomic detection, pathway identification, and experimental verification
Abstract
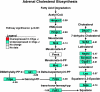
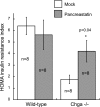
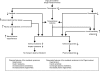
Chromogranin A (CHGA) has a crucial role in formation of regulated secretory granules in neuroendocrine tissues and is also a prohormone that is proteolytically processed into peptides with diverse and complex actions. CHGA and several of its peptide products, including catestatin and pancreastatin, are implicated in pathogenesis of essential hypertension, insulin resistance, and the metabolic syndrome. The Chga knockout mouse (Chga KO) displays severe hypertension coupled with reduction in size, number, and density of regulated secretory granules. We performed genome-wide transcriptome profiling in Chga KO adrenal gland and liver for insight into biochemical and physiological systems altered in this monogenic mouse model of hypertension. Adrenal gene expression pathway prediction of enhanced insulin sensitivity (P = 0.03) in Chga KO was confirmed with glucose, insulin, and homeostasis model assessment of insulin resistance (HOMA-IR) measurements: blood glucose was normal in Chga KO, blood insulin was reduced 4.5-fold (P < 0.0001), and HOMA-IR was decreased 3.8-fold (P < 0.002). Remarkably, such observations conclusively dissociate fundamental features of the metabolic syndrome in this monogenic hypertension model. Exogenous pancreastatin treatment restored insulin sensitivity in the Chga KO to near-normal levels. Gene expression predictions of decreased adrenal cholesterol biosynthesis (P < 0.001) and increased hepatic cholesterol biosynthesis (P < 0.001) were verified with tissue total cholesterol assays: Chga KO adrenal cholesterol decreased 1.8-fold (P = 0.039) and hepatic cholesterol increased 1.8-fold (P = 0.018). Transcriptional regulatory network prediction identified sets of transcription factors that may provide insight into the unclear mechanistic links among CHGA, cholesterol, insulin sensitivity, and the metabolic syndrome. These experiments demonstrate, for the first time, that genetic variation at the CHGA locus impacts insulin sensitivity and tissue cholesterol levels in an intact, living organism. The Chga KO may constitute a unique model for studying the relationship between the CHGA locus and disease phenotypes of the metabolic syndrome.
Figures


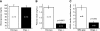
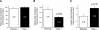
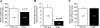
References
-
- Cadman PE, Rao F, Mahata SK, O'Connor DT. Studies of the dysglycemic peptide, pancreastatin, using a human forearm model. Ann NY Acad Sci 971: 528–529, 2002 - PubMed
-
- Chen Y, Rao F, Rodriguez-Flores JL, Mahata M, Fung MM, Stridsberg M, Vaingankar SM, Wen G, Salem RM, Das M, Cockburn MG, Schork NJ, Ziegler MG, Hamilton BA, Mahata SK, Taupenot L, O'Connor DT. Naturally occurring human genetic variation in the 3'-untranslated region of the secretory protein chromogranin A is associated with autonomic blood pressure regulation and hypertension in a sex-dependent fashion. J Am Coll Cardiol 52: 1468–1481, 2008 - PMC - PubMed
-
- Dennis G, Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol 4: P3, 2003 - PubMed
-
- Friese RS, Mahboubi P, Mahapatra NR, Mahata SK, Schork NJ, Schmid-Schoenbein GW, O'Connor DT. Neuroendocrine transcriptome in genetic hypertension: multiple changes in diverse adrenal physiological systems. Hypertension 43: 1301–1311, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

