Montelukast is a potent and durable inhibitor of multidrug resistance protein 2-mediated efflux of taxol and saquinavir
- PMID: 19952419
- PMCID: PMC2811715
- DOI: 10.1248/bpb.32.2002
Montelukast is a potent and durable inhibitor of multidrug resistance protein 2-mediated efflux of taxol and saquinavir
Abstract
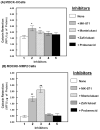
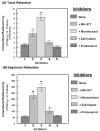
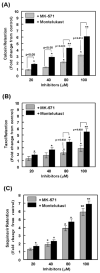
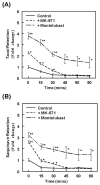
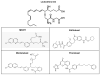
The ATP binding cassette (ABC)-transporters are energy dependent efflux pumps which regulate the pharmacokinetics of both anti-cancer chemotherapeutic agents, e.g. taxol, and of human immunodeficiency virus-1 (HIV-1) protease inhibitors (HPIs), e.g. saquinavir. Increased expression of several ABC-transporters, especially P-glycoprotein (P-gp) and multidrug resistance protein 2 (MRP2), are observed in multidrug resistant (MDR) tumor cells and on HIV-1 infected lymphocytes. In addition, due to their apical expression on vascular endothelial barriers, both P-gp and MRP2 are of crucial importance towards dictating drug access into sequestered tissues. However, although a number of P-gp inhibitors are currently in clinical trials, possible inhibitors of MRP2 are not being thoroughly investigated. The experimental leukotriene receptor antagonist (LTRA), MK-571 is known to be a potent inhibitor of MRP transporters. Using the MRP2 over-expressing Madin-Darby canine kidney cell line, MDCKII-MRP2, we evaluated whether the clinically approved LTRAs, e.g. montelukast (Singulair) and zafirlukast (Accolate), can similarly suppress MRP2-mediated efflux. We compared the efficacy of increasing concentrations (20-100 microM) of MK-571, montelukast, and zafirlukast, in suppressing the efflux of calcein-AM, a fluorescent MRP substrate, and the radiolabeled [(3)H-] drugs, taxol and saquinavir. Montelukast was the most potent inhibitor (p<0.01) of MRP2-mediated efflux of all three substrates. Montelukast also increased (p<0.01) the duration of intracellular retention of both taxol and saquinavir. More than 50% of the drugs were retained in cells even after 90 min post removal of montelukast from the medium. Our findings implicate that montelukast, a relatively safe anti-asthmatic agent, may be used as an adjunct therapy to suppress the efflux of taxol and saquinavir from MRP2 overexpressing cells.
Figures

Similar articles
-
Effects of grapefruit juice and orange juice components on P-glycoprotein- and MRP2-mediated drug efflux.Br J Pharmacol. 2004 Dec;143(7):856-64. doi: 10.1038/sj.bjp.0706008. Epub 2004 Oct 25. Br J Pharmacol. 2004. PMID: 15504753 Free PMC article.
-
Direct evidence that saquinavir is transported by multidrug resistance-associated protein (MRP1) and canalicular multispecific organic anion transporter (MRP2).Antimicrob Agents Chemother. 2002 Nov;46(11):3456-62. doi: 10.1128/AAC.46.11.3456-3462.2002. Antimicrob Agents Chemother. 2002. PMID: 12384350 Free PMC article.
-
Both P-gp and MRP2 mediate transport of Lopinavir, a protease inhibitor.Int J Pharm. 2007 Jul 18;339(1-2):139-47. doi: 10.1016/j.ijpharm.2007.02.036. Epub 2007 Mar 6. Int J Pharm. 2007. PMID: 17451894 Free PMC article.
-
MRP (ABCC) transporters-mediated efflux of anti-HIV drugs, saquinavir and zidovudine, from human endothelial cells.Exp Biol Med (Maywood). 2008 Sep;233(9):1149-60. doi: 10.3181/0802-RM-59. Epub 2008 Jun 5. Exp Biol Med (Maywood). 2008. PMID: 18535159 Free PMC article.
-
Substrates and inhibitors of human multidrug resistance associated proteins and the implications in drug development.Curr Med Chem. 2008;15(20):1981-2039. doi: 10.2174/092986708785132870. Curr Med Chem. 2008. PMID: 18691054 Review.
Cited by
-
Assessment of drug transporter function using fluorescent cell imaging.Curr Protoc Toxicol. 2013 Sep 23;57:Unit 23.6.. doi: 10.1002/0471140856.tx2306s57. Curr Protoc Toxicol. 2013. PMID: 24510579 Free PMC article.
-
Multidrug resistance proteins (MRPs/ABCCs) in cancer chemotherapy and genetic diseases.FEBS J. 2011 Sep;278(18):3226-45. doi: 10.1111/j.1742-4658.2011.08235.x. Epub 2011 Aug 1. FEBS J. 2011. PMID: 21740521 Free PMC article. Review.
-
Modulating ATP binding cassette transporters in papillary renal cell carcinoma type 2 enhances its response to targeted molecular therapy.Mol Oncol. 2018 Oct;12(10):1673-1688. doi: 10.1002/1878-0261.12346. Epub 2018 Aug 23. Mol Oncol. 2018. PMID: 29896907 Free PMC article.
-
Renal Drug Transporters and Drug Interactions.Clin Pharmacokinet. 2017 Aug;56(8):825-892. doi: 10.1007/s40262-017-0506-8. Clin Pharmacokinet. 2017. PMID: 28210973 Review.
-
Montelukast enhances cytocidal effects of carfilzomib in multiple myeloma by inhibiting mTOR pathway.Cancer Biol Ther. 2019;20(3):381-390. doi: 10.1080/15384047.2018.1529112. Epub 2018 Oct 25. Cancer Biol Ther. 2019. PMID: 30359543 Free PMC article.
References
-
- Murakami T, Takano M. Intestinal efflux transporters and drug absorption. Expert Opin Drug Metab Toxicol. 2008;4(7):923–939. - PubMed
-
- Colabufo NA, Berardi F, Contino M, Niso M, Perrone R. ABC pumps and their role in active drug transport. Curr Top Med Chem. 2009;9(2):119–129. - PubMed
-
- Loscher W, Potschka H. Drug resistance in brain diseases and the role of drug efflux transporters. Nat Rev Neurosci. 2005;6:591–602. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

