The marginating-pulmonary immune compartment in rats: characteristics of continuous inflammation and activated NK cells
- PMID: 19952959
- PMCID: PMC2797825
- DOI: 10.1097/CJI.0b013e3181b0b146
The marginating-pulmonary immune compartment in rats: characteristics of continuous inflammation and activated NK cells
Abstract
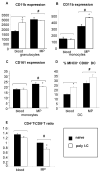
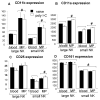
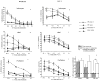
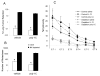
A significant role has been indicated for cellular immunity in controlling circulating cancer cells, but most autologous tumor cells seem resistant, in vitro, to natural killer cell (NKC) and cytotoxic T lymphocytes cytotoxicity. Addressing this apparent contradiction, we recently identified a unique leukocyte population, marginating-pulmonary (MP)-leukocytes, which exhibit potent natural killer (NK) cytotoxicity. Here, we characterize the MP-compartment in naive and immunostimulated rats, and assessed its cytotoxicity against "NK-resistant" tumors cells. Animals were treated with poly I-C (3x0.2 mg/kg) or saline, and circulating-leukocytes and MP-leukocytes were collected and analyzed in terms of cellular composition, cellular activation markers, and NK cytotoxicity of leukocytes and purified NKCs. Compared with circulating-leukocytes, MP-leukocytes showed greater proportion of granulocytes, monocytes, NKCs, and large NKCs; higher expression of activation and adhesion markers (CD25, CD11a, CD11b, and NKR-P1, IFN-gamma); and elevated NK cytotoxicity of leukocytes and purified NKCs against several syngeneic and xenogeneic NK-resistant target cells (from both F344 and BDX inbred rats). In immunostimulated animals (treated with poly I-C), but not in naive animals, purified NKCs from the MP-compartment showed markedly superior cytotoxicity, suggesting that poly I-C immunostimulation uniquely affect MP-NKCs, and that in naive animals other MP-leukocytes support NK cytotoxicity. Overall, the results suggest that the MP-compartment is characterized by a continuous activated inflammatory microenvironment uniquely affected by immunostimulation. If similarly potent MP-NKCs exist in patients, then circulating autologous tumor cells that are considered "NK-resistant" could actually be controlled by MP-NKCs. Innate immunity may assume greater role in controlling malignant spread, especially after immunostimulation.
Figures




Similar articles
-
The marginating-pulmonary immune compartment in mice exhibits increased NK cytotoxicity and unique cellular characteristics.Immunol Res. 2014 Jan;58(1):28-39. doi: 10.1007/s12026-013-8435-6. Immunol Res. 2014. PMID: 24132552 Free PMC article.
-
Amelioration of operation-induced suppression of marginating pulmonary NK activity using poly IC: a potential approach to reduce postoperative metastasis.Ann Surg Oncol. 2007 Feb;14(2):841-52. doi: 10.1245/s10434-006-9078-9. Epub 2006 Nov 8. Ann Surg Oncol. 2007. PMID: 17091332
-
Marginating pulmonary-NK activity and resistance to experimental tumor metastasis: suppression by surgery and the prophylactic use of a beta-adrenergic antagonist and a prostaglandin synthesis inhibitor.Brain Behav Immun. 2005 Mar;19(2):114-26. doi: 10.1016/j.bbi.2004.07.004. Brain Behav Immun. 2005. PMID: 15664784
-
Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis.Annu Rev Immunol. 2001;19:197-223. doi: 10.1146/annurev.immunol.19.1.197. Annu Rev Immunol. 2001. PMID: 11244035 Review.
-
Dual effects of cytokines in regulation of MHC-unrestricted cell mediated cytotoxicity.Crit Rev Immunol. 1993;13(1):1-34. Crit Rev Immunol. 1993. PMID: 8466640 Review.
Cited by
-
Tumor Excision as a Metastatic Russian Roulette: Perioperative Interventions to Improve Long-Term Survival of Cancer Patients.Trends Cancer. 2020 Nov;6(11):951-959. doi: 10.1016/j.trecan.2020.06.004. Epub 2020 Jul 10. Trends Cancer. 2020. PMID: 32654993 Free PMC article. Review.
-
Role of stress in the pathogenesis of cancer (Review).Int J Oncol. 2023 Nov;63(5):124. doi: 10.3892/ijo.2023.5572. Epub 2023 Sep 15. Int J Oncol. 2023. PMID: 37711028 Free PMC article. Review.
-
Selective Harvesting of Marginating-hepatic Leukocytes.J Vis Exp. 2016 Jul 21;(113):10.3791/53918. doi: 10.3791/53918. J Vis Exp. 2016. PMID: 27500423 Free PMC article.
-
Selective Harvesting of Marginating-pulmonary Leukocytes.J Vis Exp. 2016 Mar 11;(109):53849. doi: 10.3791/53849. J Vis Exp. 2016. PMID: 27023665 Free PMC article.
-
PGE2 suppresses NK activity in vivo directly and through adrenal hormones: effects that cannot be reflected by ex vivo assessment of NK cytotoxicity.Brain Behav Immun. 2013 Feb;28:128-38. doi: 10.1016/j.bbi.2012.11.003. Epub 2012 Nov 12. Brain Behav Immun. 2013. PMID: 23153554 Free PMC article.
References
-
- Shakhar G, Ben-Eliyahu S. Potential prophylactic measures against postoperative immunosuppression: could they reduce recurrence rates in oncological patients? Ann Surg Oncol. 2003;10(8):972–92. - PubMed
-
- Smyth MJ, Godfrey DI, Trapani JA. A fresh look at tumor immunosurveillance and immunotherapy. Nat Immunol. 2001;2(4):293–9. - PubMed
-
- Moretta L, et al. Human natural killer cells: Molecular mechanisms controlling NK cell activation and tumor cell lysis. Immunol Lett. 2005;100(1):7–13. - PubMed
-
- Brittenden J, et al. Natural killer cells and cancer. Cancer. 1996;77(7):1226–43. - PubMed
-
- Cerwenka A, Lanier LL. Natural killer cells, viruses and cancer. Nat Rev Immunol. 2001;1(1):41–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

