Brain distribution of carboxy terminus of Hsc70-interacting protein (CHIP) and its nuclear translocation in cultured cortical neurons following heat stress or oxygen-glucose deprivation
- PMID: 19953350
- PMCID: PMC3006630
- DOI: 10.1007/s12192-009-0162-5
Brain distribution of carboxy terminus of Hsc70-interacting protein (CHIP) and its nuclear translocation in cultured cortical neurons following heat stress or oxygen-glucose deprivation
Abstract
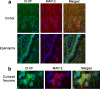
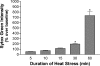
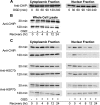
Carboxy terminus of Hsc70-interacting protein (CHIP) is thought to be a cytoprotective protein with protein quality control roles in neurodegenerative diseases and myocardial ischemia. This study describes the localization of CHIP expression in normal rodent brain and the early CHIP response in primary cultures of cortical neurons following ischemic stress models: heat stress (HS) and oxygen-glucose deprivation (OGD). CHIP was highly expressed throughout the brain, predominantly in neurons. The staining pattern was primarily cytoplasmic, although small amounts were seen in the nucleus. More intense nuclear staining was observed in primary cultured neurons which increased with stress. Nuclear accumulation of CHIP occurred within 5-10 min of HS and decreased to baseline levels or lower by 30-60 min. Decrease in nuclear CHIP at 30-60 min of HS was associated with a sharp increase in delayed cell death. While no changes in cytoplasmic CHIP were observed immediately following OGD, nuclear levels of CHIP increased slightly in response to OGD durations of 30 to 240 min. OGD-induced increases in nuclear CHIP decreased slowly during post-ischemic recovery. Nuclear CHIP decreased earlier in recovery following 120 min of OGD (4 h) than 30 min of OGD (12 h). Significant cell death first appeared between 12 and 24 h after OGD, again suggesting that delayed cell death follows closely behind the disappearance of nuclear CHIP. The ability of CHIP to translocate to and accumulate in the nucleus may be a limiting variable that determines how effectively cells respond to external stressors to facilitate cell survival. Using primary neuronal cell cultures, we were able to demonstrate rapid translocation of CHIP to the nucleus within minutes of heat stress and oxygen-glucose deprivation. An inverse relationship between nuclear CHIP and delayed cell death at 24 h suggests that the decrease in nuclear CHIP following extreme stress is linked to delayed cell death. Our findings of acute changes in subcellular localization of CHIP in response to cellular stress suggest that cellular changes that occur shortly after exposure to stress ultimately impact on the capacity and capability of a cell to recover and survive.
Figures



Similar articles
-
Oxygen glucose deprivation post-conditioning protects cortical neurons against oxygen-glucose deprivation injury: role of HSP70 and inhibition of apoptosis.J Huazhong Univ Sci Technolog Med Sci. 2014 Feb;34(1):18-22. doi: 10.1007/s11596-014-1225-0. Epub 2014 Feb 6. J Huazhong Univ Sci Technolog Med Sci. 2014. PMID: 24496673
-
CHIP Is an Essential Determinant of Neuronal Mitochondrial Stress Signaling.Antioxid Redox Signal. 2015 Aug 20;23(6):535-49. doi: 10.1089/ars.2014.6102. Epub 2015 Mar 18. Antioxid Redox Signal. 2015. PMID: 25602369 Free PMC article.
-
Selective 14-3-3γ induction quenches p-β-catenin Ser37/Bax-enhanced cell death in cerebral cortical neurons during ischemia.Cell Death Dis. 2014 Apr 17;5(4):e1184. doi: 10.1038/cddis.2014.152. Cell Death Dis. 2014. PMID: 24743739 Free PMC article.
-
Sulforaphane (SFA) protects neuronal cells from oxygen & glucose deprivation (OGD).PLoS One. 2021 Mar 18;16(3):e0248777. doi: 10.1371/journal.pone.0248777. eCollection 2021. PLoS One. 2021. PMID: 33735260 Free PMC article.
-
Neuronal Preconditioning Requires the Mitophagic Activity of C-terminus of HSC70-Interacting Protein.J Neurosci. 2018 Aug 1;38(31):6825-6840. doi: 10.1523/JNEUROSCI.0699-18.2018. Epub 2018 Jun 22. J Neurosci. 2018. PMID: 29934347 Free PMC article.
Cited by
-
C-terminus of heat shock cognate 70 interacting protein increases following stroke and impairs survival against acute oxidative stress.Antioxid Redox Signal. 2011 May 15;14(10):1787-801. doi: 10.1089/ars.2010.3300. Epub 2010 Dec 2. Antioxid Redox Signal. 2011. PMID: 20677910 Free PMC article.
-
The E3 ligase CHIP: insights into its structure and regulation.Biomed Res Int. 2014;2014:918183. doi: 10.1155/2014/918183. Epub 2014 Apr 24. Biomed Res Int. 2014. PMID: 24868554 Free PMC article. Review.
-
CHIP ameliorates neuronal damage in H2O2-induced oxidative stress in HT22 cells and gerbil ischemia.Sci Rep. 2022 Nov 30;12(1):20659. doi: 10.1038/s41598-022-22766-0. Sci Rep. 2022. PMID: 36450819 Free PMC article.
-
The chaperone-assisted selective autophagy complex dynamics and dysfunctions.Autophagy. 2023 Jun;19(6):1619-1641. doi: 10.1080/15548627.2022.2160564. Epub 2023 Jan 3. Autophagy. 2023. PMID: 36594740 Free PMC article.
-
Covalent ISG15 conjugation to CHIP promotes its ubiquitin E3 ligase activity and inhibits lung cancer cell growth in response to type I interferon.Cell Death Dis. 2018 Jan 24;9(2):97. doi: 10.1038/s41419-017-0138-9. Cell Death Dis. 2018. PMID: 29367604 Free PMC article.
References
-
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current protocols in molecular biology. New York: John Wiley & Sons; 1987.
-
- Chen J, Simon R. Ischemic tolerance in the brain. Neurology. 1997;48:306–311. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

