pH-dependent stability of neuroserpin is mediated by histidines 119 and 138; implications for the control of beta-sheet A and polymerization
- PMID: 19953505
- PMCID: PMC2865726
- DOI: 10.1002/pro.299
pH-dependent stability of neuroserpin is mediated by histidines 119 and 138; implications for the control of beta-sheet A and polymerization
Abstract
Neuroserpin is a member of the serpin superfamily. Point mutations in the neuroserpin gene underlie the autosomal dominant dementia, familial encephalopathy with neuroserpin inclusion bodies. This is characterized by the retention of ordered polymers of neuroserpin within the endoplasmic reticulum of neurons. pH has been shown to affect the propensity of several serpins to form polymers. In particular, low pH favors the formation of polymers of both alpha(1)-antitrypsin and antithrombin. We report here opposite effects in neuroserpin, with a striking resistance to polymer formation at acidic pH. Mutation of specific histidine residues showed that this effect is not attributable to the shutter domain histidine as would be predicted by analogy with other serpins. Indeed, mutation of the shutter domain His338 decreased neuroserpin stability but had no effect on the pH dependence of polymerization when compared with the wild-type protein. In contrast, mutation of His119 or His138 reduced the polymerization of neuroserpin at both acidic and neutral pH. These residues are at the lower pole of neuroserpin and provide a novel mechanism to control the opening of beta-sheet A and hence polymerization. This mechanism is likely to have evolved to protect neuroserpin from the acidic environment of the secretory granules.
Figures


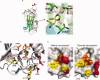

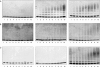
Similar articles
-
Mutant Neuroserpin (S49P) that causes familial encephalopathy with neuroserpin inclusion bodies is a poor proteinase inhibitor and readily forms polymers in vitro.J Biol Chem. 2002 May 10;277(19):17367-73. doi: 10.1074/jbc.M200680200. Epub 2002 Mar 5. J Biol Chem. 2002. PMID: 11880376
-
The serpinopathies studying serpin polymerization in vivo.Methods Enzymol. 2011;501:421-66. doi: 10.1016/B978-0-12-385950-1.00018-3. Methods Enzymol. 2011. PMID: 22078544
-
Mutants of neuroserpin that cause dementia accumulate as polymers within the endoplasmic reticulum.J Biol Chem. 2004 Jul 2;279(27):28283-91. doi: 10.1074/jbc.M313166200. Epub 2004 Apr 16. J Biol Chem. 2004. PMID: 15090543
-
Neuroserpin: a serpin to think about.Cell Mol Life Sci. 2006 Mar;63(6):709-22. doi: 10.1007/s00018-005-5077-4. Cell Mol Life Sci. 2006. PMID: 16465451 Free PMC article. Review.
-
Characterisation of serpin polymers in vitro and in vivo.Methods. 2011 Mar;53(3):255-66. doi: 10.1016/j.ymeth.2010.11.008. Epub 2010 Nov 27. Methods. 2011. PMID: 21115126 Review.
Cited by
-
Reactive centre loop dynamics and serpin specificity.Sci Rep. 2019 Mar 7;9(1):3870. doi: 10.1038/s41598-019-40432-w. Sci Rep. 2019. PMID: 30846766 Free PMC article.
-
Neuroserpin, a crucial regulator for axogenesis, synaptic modelling and cell-cell interactions in the pathophysiology of neurological disease.Cell Mol Life Sci. 2022 Mar 4;79(3):172. doi: 10.1007/s00018-022-04185-6. Cell Mol Life Sci. 2022. PMID: 35244780 Free PMC article. Review.
-
Physiological and pathological roles of tissue plasminogen activator and its inhibitor neuroserpin in the nervous system.Front Cell Neurosci. 2015 Oct 13;9:396. doi: 10.3389/fncel.2015.00396. eCollection 2015. Front Cell Neurosci. 2015. PMID: 26528129 Free PMC article. Review.
-
The tempered polymerization of human neuroserpin.PLoS One. 2012;7(3):e32444. doi: 10.1371/journal.pone.0032444. Epub 2012 Mar 6. PLoS One. 2012. PMID: 22412873 Free PMC article.
-
Functional and dysfunctional conformers of human neuroserpin characterized by optical spectroscopies and Molecular Dynamics.Biochim Biophys Acta. 2015 Feb;1854(2):110-7. doi: 10.1016/j.bbapap.2014.10.002. Epub 2014 Nov 6. Biochim Biophys Acta. 2015. PMID: 25450507 Free PMC article.
References
-
- Silverman GA, Bird PI, Carrell RW, Coughlin PB, Gettins PG, Irving JI, Lomas DA, Luke CJ, Moyer RW, Pemberton PA, Remold-O'Donnell E, Salvesen GS, Travis J, Whisstock JC. The serpins are an expanding superfamily of structurally similar but functionally diverse proteins: evolution, mechanism of inhibition, novel functions, and a revised nomenclature. J Biol Chem. 2001;276:33293–33296. - PubMed
-
- Galliciotti G, Sonderegger P. Neuroserpin. Front Biosci. 2006;11:33–45. - PubMed
-
- Huntington JA. Shape-shifting serpins—advantages of a mobile mechanism. Trends Biochem Sci. 2006;31:427–435. - PubMed
-
- Hastings GA, Coleman TA, Haudenschild CC, Stefansson S, Smith EP, Barthlow R, Cherry S, Sandkvist M, Lawrence DA. Neuroserpin, a brain-associated inhibitor of tissue plasminogen activator is localized primarily in neurons. Implications for the regulation of motor learning and neuronal survival. J Biol Chem. 1997;272:33062–33067. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

