One-pot catalytic enantio- and diastereoselective syntheses of anti-, syn-cis-disubstituted, and syn-vinyl cyclopropyl alcohols
- PMID: 19954146
- PMCID: PMC2805046
- DOI: 10.1021/ja907781t
One-pot catalytic enantio- and diastereoselective syntheses of anti-, syn-cis-disubstituted, and syn-vinyl cyclopropyl alcohols
Abstract
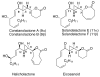
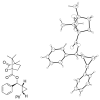
Highly enantio- and diastereoselective methods for the synthesis of a variety of cyclopropyl alcohols are reported. These methods represent the first one-pot approaches to syn-vinyl cyclopropyl alcohols, syn-cis-disubstituted cyclopropyl alcohols, and anti-cyclopropyl alcohols from achiral precursors. The methods begin with enantioselective C-C bond formations promoted by a MIB-based zinc catalyst to generate allylic alkoxide intermediates. The intermediates are then subjected to in situ alkoxide-directed cyclopropanation to provide cyclopropyl alcohols. In the synthesis of vinyl cyclopropyl alcohols, hydroboration of enynes is followed by transmetalation of the resulting dienylborane to zinc to provide dienylzinc reagents. Enantioselective addition to aldehydes generates the requisite dienyl zinc alkoxides, which are then subjected to in situ cyclopropanation to furnish vinyl cyclopropyl alcohols. Cyclopropanation occurs at the double bond allylic to the alkoxide. Using this method, syn-vinylcyclopropyl alcohols are obtained in 65-85% yield, 76-93% ee, and > 19:1 dr. To prepare anti-cyclopropanols, enantioselective addition of alkylzinc reagents to conjugated enals provides allylic zinc alkoxides. Because direct cyclopropanation provides syn-cyclopropyl alcohols, the intermediate allylic alkoxides were treated with TMSCl/Et(3)N to generate intermediate silyl ethers. In situ cyclopropanation of the allylic silyl ether resulted in cyclopropanation to form the anti-cyclopropyl silyl ether. Workup with TBAF affords the anti-cyclopropyl alcohols in one pot in 60-82% yield, 89-99% ee, and > or = 10:1 dr. For the synthesis of cis-disubstituted cyclopropyl alcohols, in situ generated (Z)-vinyl zinc reagents were employed in asymmetric addition to aldehydes to generate (Z)-allylic zinc alkoxides. In situ cyclopropanation provides syn-cis-disubstituted cyclopropyl alcohols in 42-70% yield, 88-97% ee, and > 19:1 dr. These one-pot procedures enable the synthesis of a diverse array of cyclopropyl alcohol building blocks with high enantio- and diastereoselectivities.
Figures




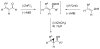

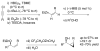
Similar articles
-
Efficient approaches to the stereoselective synthesis of cyclopropyl alcohols.Acc Chem Res. 2012 Sep 18;45(9):1533-47. doi: 10.1021/ar300052s. Epub 2012 Jun 22. Acc Chem Res. 2012. PMID: 22725974
-
Highly enantio- and diastereoselective one-pot methods for the synthesis of halocyclopropyl alcohols.J Am Chem Soc. 2009 Jan 28;131(3):954-62. doi: 10.1021/ja806989n. J Am Chem Soc. 2009. PMID: 19154172 Free PMC article.
-
Catalytic asymmetric generation of (Z)-disubstituted allylic alcohols.J Am Chem Soc. 2007 Dec 26;129(51):16119-25. doi: 10.1021/ja0762285. Epub 2007 Dec 4. J Am Chem Soc. 2007. PMID: 18052173 Free PMC article.
-
Tandem reactions for streamlining synthesis: enantio- and diastereoselective one-pot generation of functionalized epoxy alcohols.Acc Chem Res. 2008 Aug;41(8):883-93. doi: 10.1021/ar800006h. Acc Chem Res. 2008. PMID: 18710197 Free PMC article. Review.
-
Asymmetric functional organozinc additions to aldehydes catalyzed by 1,1'-bi-2-naphthols (BINOLs).Acc Chem Res. 2014 May 20;47(5):1523-35. doi: 10.1021/ar500020k. Epub 2014 Apr 16. Acc Chem Res. 2014. PMID: 24738985 Free PMC article. Review.
Cited by
-
Stereoselective synthesis of β-hydroxy enamines, aminocyclopropanes, and 1,3-amino alcohols via asymmetric catalysis.J Am Chem Soc. 2010 Oct 13;132(40):14179-90. doi: 10.1021/ja105435y. J Am Chem Soc. 2010. PMID: 20853837 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

