Concentration and purification of human immunodeficiency virus type 1 virions by microfluidic separation of superparamagnetic nanoparticles
- PMID: 19954210
- PMCID: PMC2818585
- DOI: 10.1021/ac9024522
Concentration and purification of human immunodeficiency virus type 1 virions by microfluidic separation of superparamagnetic nanoparticles
Abstract
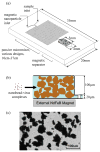
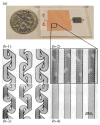
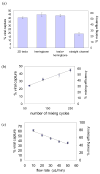
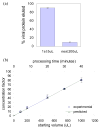
The low concentration and complex sample matrix of many clinical and environmental viral samples presents a significant challenge in the development of low cost, point-of-care viral assays. To address this problem, we investigated the use of a microfluidic passive magnetic separator combined with on-chip mixer to both purify and concentrate whole-particle human immunodeficiency virus type 1 (HIV-1) virions. Virus-containing plasma samples are first mixed to allow specific binding of the viral particles with antibody-conjugated superparamagnetic nanoparticles, and several passive mixer geometries were assessed for their mixing efficiencies. The virus-nanoparticle complexes are then separated from the plasma in a novel magnetic separation chamber, where packed micrometer-sized ferromagnetic particles serve as high magnetic gradient concentrators for an externally applied magnetic field. Thereafter, a viral lysis buffer was flowed through the chip and the released HIV proteins were assayed off-chip. Viral protein extraction efficiencies of 62% and 45% were achieved at 10 and 30 muL/min throughputs, respectively. More importantly, an 80-fold concentration was observed for an initial sample volume of 1 mL and a 44-fold concentration for an initial sample volume of 0.5 mL. The system is broadly applicable to microscale sample preparation of any viral sample and can be used for nucleic acid extraction as well as 40-80-fold enrichment of target viruses.
Figures

Similar articles
-
Quantum dot-based HIV capture and imaging in a microfluidic channel.Biosens Bioelectron. 2009 Sep 15;25(1):253-8. doi: 10.1016/j.bios.2009.06.023. Epub 2009 Jun 18. Biosens Bioelectron. 2009. PMID: 19665685 Free PMC article.
-
Nanoparticle labelling-based magnetic immunoassay on chip combined with electrothermal vaporization-inductively coupled plasma mass spectrometry for the determination of carcinoembryonic antigen in human serum.Analyst. 2011 Oct 7;136(19):3934-42. doi: 10.1039/c1an15387k. Epub 2011 Aug 22. Analyst. 2011. PMID: 21858374
-
On-chip manipulation of continuous picoliter-volume superparamagnetic droplets using a magnetic force.Lab Chip. 2009 Oct 21;9(20):2992-9. doi: 10.1039/b906229g. Epub 2009 Jul 9. Lab Chip. 2009. PMID: 19789755
-
Magnetoanalysis of micro/nanoparticles: a review.Anal Chim Acta. 2011 Apr 1;690(2):137-47. doi: 10.1016/j.aca.2011.02.019. Epub 2011 Feb 12. Anal Chim Acta. 2011. PMID: 21435469 Review.
-
Analytical methods for separating and isolating magnetic nanoparticles.Phys Chem Chem Phys. 2012 Mar 14;14(10):3280-9. doi: 10.1039/c2cp22982j. Epub 2012 Feb 3. Phys Chem Chem Phys. 2012. PMID: 22306911 Review.
Cited by
-
Synthesis and application of superabsorbent polymer microspheres for rapid concentration and quantification of microbial pathogens in ambient water.Sep Purif Technol. 2020 May 15;239:116540. doi: 10.1016/j.seppur.2020.116540. Sep Purif Technol. 2020. PMID: 32421015 Free PMC article.
-
"Nanofiltration" Enabled by Super-Absorbent Polymer Beads for Concentrating Microorganisms in Water Samples.Sci Rep. 2016 Feb 15;6:20516. doi: 10.1038/srep20516. Sci Rep. 2016. PMID: 26876979 Free PMC article.
-
Advances in nanomaterials and their applications in point of care (POC) devices for the diagnosis of infectious diseases.Biotechnol Adv. 2016 Dec;34(8):1275-1288. doi: 10.1016/j.biotechadv.2016.09.003. Epub 2016 Sep 26. Biotechnol Adv. 2016. PMID: 27686397 Free PMC article. Review.
-
Multiple virus sorting based on aptamer-modified microspheres in a TSAW device.Microsyst Nanoeng. 2023 May 17;9:64. doi: 10.1038/s41378-023-00523-1. eCollection 2023. Microsyst Nanoeng. 2023. PMID: 37213822 Free PMC article.
-
Micro and Nanoscale Technologies for Diagnosis of Viral Infections.Small. 2021 Nov;17(45):e2100692. doi: 10.1002/smll.202100692. Epub 2021 Jul 26. Small. 2021. PMID: 34310048 Free PMC article. Review.
References
-
- Global summary of the AIDS epidemic 2008. World Health Oraganization; 2008. [accessed 7th April 2009]. available at http://www.who.int/hiv/data/2008_global_summary_AIDS_ep.png.
-
- Calmy A, Ford N, Hirschel B, Reynolds SJ, Lynen L, Goemaere E, Garcia de la Vega F, Perrin L, Rodriguez W. Clin Infect Dis. 2007;44:128. - PubMed
-
- Rouet F, Rouzioux C. Clinical Laboratory. 2007;53:135. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

