Synthesis and in vitro opioid receptor functional antagonism of methyl-substituted analogues of (3R)-7-hydroxy-N-[(1S)-1-{[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperidinyl]methyl}-2-methylpropyl]-1,2,3,4-tetrahydro-3-isoquinolinecarboxamide (JDTic)
- PMID: 19954245
- PMCID: PMC5584631
- DOI: 10.1021/jm900756t
Synthesis and in vitro opioid receptor functional antagonism of methyl-substituted analogues of (3R)-7-hydroxy-N-[(1S)-1-{[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperidinyl]methyl}-2-methylpropyl]-1,2,3,4-tetrahydro-3-isoquinolinecarboxamide (JDTic)
Abstract
In previous structure-activity relationship (SAR) studies, (3R)-7-hydroxy-N-[(1S)-1-{[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperidinyl]methyl}-2-methylpropyl]-1,2,3,4-tetrahydro-3-isoquinolinecarboxamide (JDTic, 3) was identified as the first potent and selective kappa-opioid receptor antagonist from the trans-3,4-dimethyl-4-(3-hydroxyphenyl)piperidine class of opioid antagonists. In the present study, we report the synthesis of analogues 8a-p of 3 and present their in vitro opioid receptor functional antagonism using a [(35)S]GTPgammaS binding assay. Compounds 8a-p are analogues of 3 containing one, two, or three methyl groups connected to the JDTic structure at five different positions. All the analogues with one and two added methyl groups with the exception of 8k had subnanomolar K(e) values at the kappa receptor. The three most potent analogues were the monomethylated (3R)-7-hydroxy-N-[(1S,2S)-1-{[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethylpiperidine-1-yl]methyl}-2-methylbutyl]-1,2,3,4-tetrahydroisoquinoline-3-carboxamide (8a) and (3R)-7-hydroxy-N-[(1S)-1-{[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethylpiperidin-1-yl]methyl}-(2-methylpropyl)]-3-methyl-1,2,3,4-tetrahydroisoquinoline-3-carboxamide (8e) with K(e) values of 0.03 nM at the kappa receptor and (3R)-7-hydroxy-N-[(1S)-1-{[(3R,4R)-4-(3-methoxyphenyl)-3,4-dimethylpiperidin-1-yl]methyl}-2-methylpropyl]-1,2,3,4-tetrahydroisoquinoline-3-carboxamide (8d) with K(e) = 0.037 nM at the kappa receptor. All three compounds were selective for the kappa receptor relative to the micro and delta receptors. Overall, the results from this study highlight those areas that are tolerant to substitution on 3.
Figures
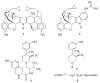
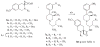
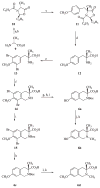
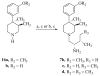
References
-
- Volkow ND, Li TK. Drug addiction: the neurobiology of behaviour gone awry. Nat Rev Neurosci. 2004;5:963–970. - PubMed
-
- Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. - PubMed
-
- Pfeiffer A, Brantl V, Herz A, Emrich HM. Psychotomimesis mediated by kappa opiate receptors. Science. 1986;233:774–776. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

