Synthesis, properties, and applications of diazotrifluropropanoyl-containing photoactive analogs of farnesyl diphosphate containing modified linkages for enhanced stability
- PMID: 19954434
- PMCID: PMC2836812
- DOI: 10.1111/j.1747-0285.2009.00914.x
Synthesis, properties, and applications of diazotrifluropropanoyl-containing photoactive analogs of farnesyl diphosphate containing modified linkages for enhanced stability
Abstract
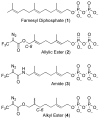
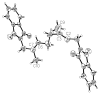
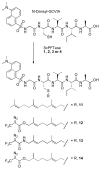
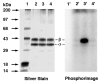
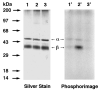
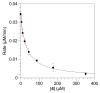
Photoactive analogs of farnesyl diphosphate (FPP) are useful probes in studies of enzymes that employ this molecule as a substrate. Here, we describe the preparation and properties of two new FPP analogs that contain diazotrifluoropropanoyl photophores linked to geranyl diphosphate via amide or ester linkages. The amide-linked analog (3) was synthesized in 32P-labeled form from geraniol in seven steps. Experiments with Saccharomyces cerevisiae protein farnesyltransferase (ScPFTase) showed that 3 is an alternative substrate for the enzyme. Photolysis experiments with [(32)P]3 demonstrate that this compound labels the beta-subunits of both farnesyltransferase and geranylgeranyltransferase (types 1 and 2). However, the amide-linked probe 3 undergoes a rearrangement to a photochemically unreactive isomeric triazolone upon long term storage making it inconvenient to use. To address this stability issue, the ester-linked analog 4 was prepared in six steps from geraniol. Computational analysis and X-ray crystallographic studies suggest that 4 binds to protein farnesyl transferase (PFTase) in a similar fashion as FPP. Compound 4 is also an alternative substrate for PFTase, and a 32P-labeled form selectively photocrosslinks the beta-subunit of ScPFTase as well as E. coli farnesyldiphosphate synthase and a germacrene-producing sesquiterpene synthase from Nostoc sp. strain PCC7120 (a cyanobacterial source). Finally, nearly exclusive labeling of ScPFTase in crude E. coli extract was observed, suggesting that [32P]4 manifests significant selectivity and should hence be useful for identifying novel FPP-utilizing enzymes in crude protein preparations.
Figures
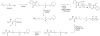

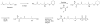
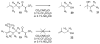




Similar articles
-
A versatile photoactivatable probe designed to label the diphosphate binding site of farnesyl diphosphate utilizing enzymes.Bioorg Med Chem. 2009 Jul 1;17(13):4797-805. doi: 10.1016/j.bmc.2009.04.034. Epub 2009 Apr 22. Bioorg Med Chem. 2009. PMID: 19447628 Free PMC article.
-
Photoactive analogs of farnesyl diphosphate and related isoprenoids: design and applications in studies of medicinally important isoprenoid-utilizing enzymes.Curr Med Chem. 2013;20(12):1585-94. doi: 10.2174/0929867311320120008. Curr Med Chem. 2013. PMID: 23409719 Review.
-
Biochemical and structural studies with prenyl diphosphate analogues provide insights into isoprenoid recognition by protein farnesyl transferase.Biochemistry. 2003 Apr 8;42(13):3716-24. doi: 10.1021/bi0266838. Biochemistry. 2003. PMID: 12667062
-
Synthesis of farnesyl diphosphate analogues containing ether-linked photoactive benzophenones and their application in studies of protein prenyltransferases.J Org Chem. 2001 May 18;66(10):3253-64. doi: 10.1021/jo991130x. J Org Chem. 2001. PMID: 11348105
-
Photoaffinity analogues of farnesyl pyrophosphate transferable by protein farnesyl transferase.J Am Chem Soc. 2002 Jul 17;124(28):8206-19. doi: 10.1021/ja0124717. J Am Chem Soc. 2002. PMID: 12105898
Cited by
-
Protein prenylation: enzymes, therapeutics, and biotechnology applications.ACS Chem Biol. 2015 Jan 16;10(1):51-62. doi: 10.1021/cb500791f. Epub 2014 Dec 8. ACS Chem Biol. 2015. PMID: 25402849 Free PMC article. Review.
-
Mutation of archaeal isopentenyl phosphate kinase highlights mechanism and guides phosphorylation of additional isoprenoid monophosphates.ACS Chem Biol. 2010 Jun 18;5(6):589-601. doi: 10.1021/cb1000313. ACS Chem Biol. 2010. PMID: 20392112 Free PMC article.
-
A Not-So-Ancient Grease History: Click Chemistry and Protein Lipid Modifications.Chem Rev. 2021 Jun 23;121(12):7178-7248. doi: 10.1021/acs.chemrev.0c01108. Epub 2021 Apr 6. Chem Rev. 2021. PMID: 33821625 Free PMC article. Review.
-
Photoaffinity labeling of Ras converting enzyme 1 (Rce1p) using a benzophenone-containing peptide substrate.Bioorg Med Chem. 2010 Aug 1;18(15):5675-84. doi: 10.1016/j.bmc.2010.06.024. Epub 2010 Jun 12. Bioorg Med Chem. 2010. PMID: 20619662 Free PMC article.
-
Evaluation of alkyne-modified isoprenoids as chemical reporters of protein prenylation.Chem Biol Drug Des. 2010 Dec;76(6):460-71. doi: 10.1111/j.1747-0285.2010.01037.x. Epub 2010 Oct 11. Chem Biol Drug Des. 2010. PMID: 21040496 Free PMC article.
References
-
- Cane DE. Sesquiterpene biosynthesis: Cyclization mechanisms. Comp Nat Prod Chem. 1999;2:155–200.
-
- Davis EM, Croteau R. Cyclization enzymes in the biosynthesis of monoterpenes, sesquiterpenes, and diterpenes. Topics Curr Chem. 2000;209:53–95.
-
- Loomis WD, Croteau R. Biochemistry of terpenoids. Biochem Plants. 1980;4:363–418.
-
- Zhang FL, Casey PJ. Protein prenylation: Molecular mechanisms and functional consequences. Ann Rev Biochem. 1996;65:241–69. - PubMed
-
- Edelstein RL, Distefano MD. Photoaffinity labeling of yeast farnesyl protein transferase and enzymic synthesis of a Ras protein incorporating a photoactive isoprenoid. Biochem Biophys Res Comm. 1997;235:377–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

