Gamma motor neurons express distinct genetic markers at birth and require muscle spindle-derived GDNF for postnatal survival
- PMID: 19954518
- PMCID: PMC2800842
- DOI: 10.1186/1749-8104-4-42
Gamma motor neurons express distinct genetic markers at birth and require muscle spindle-derived GDNF for postnatal survival
Abstract
Background: Gamma motor neurons (gamma-MNs) selectively innervate muscle spindle intrafusal fibers and regulate their sensitivity to stretch. They constitute a distinct subpopulation that differs in morphology, physiology and connectivity from alpha-MNs, which innervate extrafusal muscle fibers and exert force. The mechanisms that control the differentiation of functionally distinct fusimotor neurons are unknown. Progress on this question has been limited by the absence of molecular markers to specifically distinguish and manipulate gamma-MNs. Recently, it was reported that early embryonic gamma-MN precursors are dependent on GDNF. Using this knowledge we characterized genetic strategies to label developing gamma-MNs based on GDNF receptor expression, showed their strict dependence for survival on muscle spindle-derived GDNF and generated an animal model in which gamma-MNs are selectively lost.
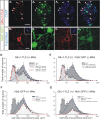
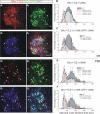
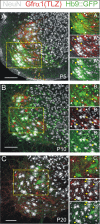
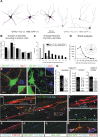
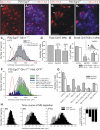
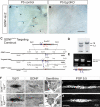
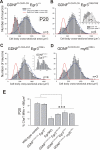
Results: In mice heterozygous for both the Hb9::GFP transgene and a tau-lacZ-labeled (TLZ) allele of the GDNF receptor Gfralpha1, we demonstrated that small motor neurons with high Gfralpha1-TLZ expression and lacking Hb9::GFP display structural and synaptic features of gamma-MNs and are selectively lost in mutants lacking target muscle spindles. Loss of muscle spindles also results in the downregulation of Gfralpha1 expression in some large diameter MNs, suggesting that spindle-derived factors may also influence populations of alpha-MNs with beta-skeletofusimotor collaterals. These molecular markers can be used to identify gamma-MNs from birth to the adult and to distinguish gamma- from beta-motor axons in the periphery. We also found that postnatal gamma-MNs are also distinguished by low expression of the neuronal nuclear protein (NeuN). With these markers of gamma-MN identity, we show after conditional elimination of GDNF from muscle spindles that the survival of gamma-MNs is selectively dependent on spindle-derived GDNF during the first 2 weeks of postnatal development.
Conclusion: Neonatal gamma-MNs display a unique molecular profile characterized by the differential expression of a series of markers - Gfralpha1, Hb9::GFP and NeuN - and the selective dependence on muscle spindle-derived GDNF. Deletion of GDNF expression from muscle spindles results in the selective elimination of gamma-MNs with preservation of the spindle and its sensory innervation. This provides a mouse model with which to explore the specific role of gamma-fusimotor activity in motor behaviors.
Figures
References
-
- Hulliger M. The mammalian muscle spindle and its central control. Rev Physiol Biochem Pharmacol. 1984;101:1–110. full_text. - PubMed
-
- Laporte Y, Emonet-Denard F, Jami L. The skeleto-fusimotor or beta-innervation of mammalian muscle spinldes. TINS. 1981;4:97–99.
-
- Prochazka A. Exercise: Regulation and Integration of Multiple Systems. Volume sect. 12. Bethesda, MD: Am Physiol Soc; 1996. Proprioceptive feedback and movement regulation; pp. 89–127. Handbook of Physiology.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

