Improving the outcome of infants born at <30 weeks' gestation--a randomized controlled trial of preventative care at home
- PMID: 19954550
- PMCID: PMC2797495
- DOI: 10.1186/1471-2431-9-73
Improving the outcome of infants born at <30 weeks' gestation--a randomized controlled trial of preventative care at home
Abstract
Background: Early developmental interventions to prevent the high rate of neurodevelopmental problems in very preterm children, including cognitive, motor and behavioral impairments, are urgently needed. These interventions should be multi-faceted and include modules for caregivers given their high rates of mental health problems.
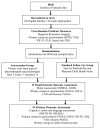
Methods/design: We have designed a randomized controlled trial to assess the effectiveness of a preventative care program delivered at home over the first 12 months of life for infants born very preterm (<30 weeks of gestational age) and their families, compared with standard medical follow-up. The aim of the program, delivered over nine sessions by a team comprising a physiotherapist and psychologist, is to improve infant development (cognitive, motor and language), behavioral regulation, caregiver-child interactions and caregiver mental health at 24 months' corrected age. The infants will be stratified by severity of brain white matter injury (assessed by magnetic resonance imaging) at term equivalent age, and then randomized. At 12 months' corrected age interim outcome measures will include motor development assessed using the Alberta Infant Motor Scale and the Neurological Sensory Motor Developmental Assessment. Caregivers will also complete a questionnaire at this time to obtain information on behavior, parenting, caregiver mental health, and social support. The primary outcomes are at 24 months' corrected age and include cognitive, motor and language development assessed with the Bayley Scales of Infant and Toddler Development (Bayley-III). Secondary outcomes at 24 months include caregiver-child interaction measured using an observational task, and infant behavior, parenting, caregiver mental health and social support measured via standardized parental questionnaires.
Discussion: This paper presents the background, study design and protocol for a randomized controlled trial in very preterm infants utilizing a preventative care program in the first year after discharge home designed to improve cognitive, motor and behavioral outcomes of very preterm children and caregiver mental health at two-years' corrected age.
Clinical trial registration number: ACTRN12605000492651.
Figures
References
-
- Marchand L, André M, Arnaud C, Pierrat V, Rozé J, Messer J, Thiriez G, Burguet A, Picaud J, Bréart G. Neurodevelopmental disabilities and special care of 5-year-old children born before 33 weeks of gestation (the EPIPAGE study): a longitudinal cohort study. The Lancet. 2008;371(9615):813. doi: 10.1016/S0140-6736(08)60380-3. - DOI - PubMed
-
- Spittle AJ, Orton J, Doyle LW, Boyd R. Early developmental intervention programs post hospital discharge to prevent motor and cognitive impairments in preterm infants. Cochrane Database Syst Rev. 2007. p. CD005495. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

