RACK1 is involved in β-amyloid impairment of muscarinic regulation of GABAergic transmission
- PMID: 19954860
- PMCID: PMC2888795
- DOI: 10.1016/j.neurobiolaging.2009.10.017
RACK1 is involved in β-amyloid impairment of muscarinic regulation of GABAergic transmission
Abstract
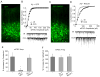
RACK1 (receptor for activated C-kinase 1), an anchoring protein that shuttles activated PKC to cellular membranes, plays an important role in PKC-mediated signal transduction pathways. A significant loss of RACK1 has been found in the brain of aging animals and Alzheimer's disease (AD) patients, which implicates the potential involvement of RACK1 in altered PKC activation associated with dementia. Our previous studies have demonstrated that GABAergic inhibition in prefrontal cortex, which is important for cognitive processes like "working memory", is regulated by muscarinic receptors via a PKC-dependent mechanism, and this effect is impaired by β-amyloid peptide (Aβ). In this study, we found that Aβ oligomers decreased RACK1 distribution in the membrane fraction of cortical neurons. Moreover, overexpression of RACK1 rescued the effect of muscarinic receptors on GABAergic transmission in Aβ-treated cortical cultures in vitro and Aβ-injected cortical neurons in vivo. These results suggest that the Aβ-induced loss of RACK1 distribution in the cell membrane may underlie the Aβ impairment of muscarinic regulation of PKC and GABAergic transmission. Thus, RACK1 provides a potential therapeutic target that can restore some of the impaired cellular processes by Aβ.
Copyright © 2009 Elsevier Inc. All rights reserved.
Figures



References
-
- Araujo DM, Lapchak PA, Robitaille Y, Gauthier S, Quirion R. Differential alteration of various cholinergic markers in cortical and subcortical regions of human brain in Alzheimer’s disease. J Neurochem. 1988;50(6):1914–1923. - PubMed
-
- Battaini F, Pascale A, Paoletti R, Govoni S. The role of anchoring protein RACK1 in PKC activation in the ageing rat brain. Trends Neurosci. 1997;20:410–415. - PubMed
-
- Battaini F, Pascale A, Lucchi L, Pasinetti GM, Govoni S. Protein kinase C anchoring deficit in postmortem brains of Alzheimer’s disease patients. Exp Neurol. 1999;159:559–564. - PubMed
-
- Behn-Krappa A, Newton AC. The hydrophobic phosphorylation motif of conventional protein kinase C is regulated by autophosphorylation. Curr Biol. 1999;9:728–37. - PubMed
-
- Broersen LM, Heinsbroek RPW, de Bruin JPC, Uylings HBM, Oliver B. The role of the medial prefrontal cortex of rats in short term memory functioning: further support for involvement of cholinergic, rather than dopaminergic mechanisms. Brain Res. 1995;674:221–229. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

