Laserspray ionization, a new atmospheric pressure MALDI method for producing highly charged gas-phase ions of peptides and proteins directly from solid solutions
- PMID: 19955086
- PMCID: PMC2830846
- DOI: 10.1074/mcp.M900527-MCP200
Laserspray ionization, a new atmospheric pressure MALDI method for producing highly charged gas-phase ions of peptides and proteins directly from solid solutions
Abstract
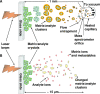
The first example of a matrix-assisted laser desorption/ionization (MALDI) process producing multiply charged mass spectra nearly identical to those observed with electrospray ionization (ESI) is presented. MALDI is noted for its ability to produce singly charged ions, but in the experiments described here multiply charged ions are produced by laser ablation of analyte incorporated into a common MALDI matrix, 2,5-dihydroxybenzoic acid, using standard solvent-based sample preparation protocols. Laser ablation is known to produce matrix clusters in MALDI provided a threshold energy is achieved. We propose that these clusters (liquid droplets) are highly charged, and under conditions that produce sufficient matrix evaporation, ions are field-evaporated from the droplets similarly to ESI. Because of the multiple charging, advanced mass spectrometers with limited mass-to-charge range can be used for protein characterization. Thus, using an Orbitrap mass spectrometer, low femtomole quantities of proteins produce full-range mass spectra at 100,000 mass resolution with <5-ppm mass accuracy and with 1-s acquisition. Furthermore, the first example of protein fragmentation using electron transfer dissociation with MALDI is presented.
Figures




References
-
- Karas M., Glückmann M., Schäfer J. ( 2000) Ionization in matrix-assisted laser desorption/ionization: singly charged molecular ions are the lucky survivors. J. Mass Spectrom 35, 1– 12 - PubMed
-
- Glückmann M., Pfenninger A., Krüger R., Thierolf M., Karas M., Horneffer V., Hillenkamp F., Strupat K. ( 2001) Mechanisms in MALDI analysis: surface interaction or incorporation of analytes? Int. J. Mass Spectrom 210/211, 121– 132
-
- Fournier I., Brunot A., Tabet J. C., Bolbach G. ( 2002) Delayed extraction experiments using a repulsive potential before ion extraction: evidence of clusters as ion precursors in UV-MALDI. Part I: dynamical effects with the matrix 2,5-dihydroxybenzoic acid. Int. J. Mass Spectrom 213, 203– 215
-
- Knochenmuss R. ( 2003) A quantitative model of ultraviolet matrix-assisted laser desorption/ionization including analyte ion generation. Anal. Chem 75, 2199– 2207 - PubMed
-
- Knochenmuss R. ( 2006) Ion formation mechanisms in UV-MALDI. Analyst 131, 966– 986 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

