Transforming growth factor beta-activated kinase 1 (TAK1) kinase adaptor, TAK1-binding protein 2, plays dual roles in TAK1 signaling by recruiting both an activator and an inhibitor of TAK1 kinase in tumor necrosis factor signaling pathway
- PMID: 19955178
- PMCID: PMC2807291
- DOI: 10.1074/jbc.M109.090522
Transforming growth factor beta-activated kinase 1 (TAK1) kinase adaptor, TAK1-binding protein 2, plays dual roles in TAK1 signaling by recruiting both an activator and an inhibitor of TAK1 kinase in tumor necrosis factor signaling pathway
Abstract
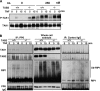
Transforming growth factor beta-activated kinase 1 (TAK1) kinase is an indispensable signaling intermediate in tumor necrosis factor (TNF), interleukin 1, and Toll-like receptor signaling pathways. TAK1-binding protein 2 (TAB2) and its closely related protein, TAB3, are binding partners of TAK1 and have previously been identified as adaptors of TAK1 that recruit TAK1 to a TNF receptor signaling complex. TAB2 and TAB3 redundantly mediate activation of TAK1. In this study, we investigated the role of TAB2 by analyzing fibroblasts having targeted deletion of the tab2 gene. In TAB2-deficient fibroblasts, TAK1 was associated with TAB3 and was activated following TNF stimulation. However, TAB2-deficient fibroblasts displayed a significantly prolonged activation of TAK1 compared with wild type control cells. This suggests that TAB2 mediates deactivation of TAK1. We found that a TAK1-negative regulator, protein phosphatase 6 (PP6), was recruited to the TAK1 complex in wild type but not in TAB2-deficient fibroblasts. Furthermore, we demonstrated that both PP6 and TAB2 interacted with the polyubiquitin chains and this interaction mediated the assembly with TAK1. Our results indicate that TAB2 not only activates TAK1 but also plays an essential role in the deactivation of TAK1 by recruiting PP6 through a polyubiquitin chain-dependent mechanism.
Figures



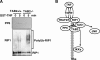
References
-
- Kawai T., Akira S. (2007) Trends Mol. Med. 13, 460–469 - PubMed
-
- Ninomiya-Tsuji J., Kishimoto K., Hiyama A., Inoue J., Cao Z., Matsumoto K. (1999) Nature 398, 252–256 - PubMed
-
- Sato S., Sanjo H., Takeda K., Ninomiya-Tsuji J., Yamamoto M., Kawai T., Matsumoto K., Takeuchi O., Akira S. (2005) Nat. Immunol. 6, 1087–1095 - PubMed
-
- Takaesu G., Surabhi R. M., Park K. J., Ninomiya-Tsuji J., Matsumoto K., Gaynor R. B. (2003) J. Mol. Biol. 326, 105–115 - PubMed
-
- Ea C. K., Deng L., Xia Z. P., Pineda G., Chen Z. J. (2006) Mol. Cell 22, 245–257 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

