Management of newly diagnosed symptomatic multiple myeloma: updated Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus guidelines
- PMID: 19955246
- PMCID: PMC2787395
- DOI: 10.4065/mcp.2009.0603
Management of newly diagnosed symptomatic multiple myeloma: updated Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus guidelines
Abstract
Multiple myeloma is a malignant plasma cell neoplasm that affects more than 20,000 people each year and is the second most common hematologic malignancy. It is part of a spectrum of monoclonal plasma cell disorders, many of which do not require active therapy. During the past decade, considerable progress has been made in our understanding of the disease process and factors that influence outcome, along with development of new drugs that are highly effective in controlling the disease and prolonging survival without compromising quality of life. Identification of well-defined and reproducible prognostic factors and introduction of new therapies with unique modes of action and impact on disease outcome have for the first time opened up the opportunity to develop risk-adapted strategies for managing this disease. Although these risk-adapted strategies have not been prospectively validated, enough evidence can be gathered from existing randomized trials, subgroup analyses, and retrospective studies to develop a working framework. This set of recommendations represents such an effort-the development of a set of consensus guidelines by a group of experts to manage patients with newly diagnosed disease based on an interpretation of the best available evidence.
Figures
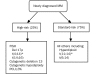
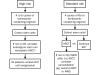


References
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225-249 - PubMed
-
- Fonseca R. Many and multiple myeloma(s). Leukemia 2003;17(10):1943-1944 - PubMed
-
- Fonseca R, Barlogie B, Bataille R, et al. Genetics and cytogenetics of multiple myeloma: a workshop report. Cancer Res. 2004;64(4):1546-1558 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA083724/CA/NCI NIH HHS/United States
- CA129966/CA/NCI NIH HHS/United States
- R01 CA136671/CA/NCI NIH HHS/United States
- R01 CA100080/CA/NCI NIH HHS/United States
- CA93842/CA/NCI NIH HHS/United States
- R01 CA093842/CA/NCI NIH HHS/United States
- P01 CA062242/CA/NCI NIH HHS/United States
- CA150831/CA/NCI NIH HHS/United States
- R01 CA125614/CA/NCI NIH HHS/United States
- CA062242/CA/NCI NIH HHS/United States
- CA100707/CA/NCI NIH HHS/United States
- CA83724/CA/NCI NIH HHS/United States
- R01 CA129966/CA/NCI NIH HHS/United States
- R01 CA100634/CA/NCI NIH HHS/United States
- R01 CA107476/CA/NCI NIH HHS/United States
- CA107476/CA/NCI NIH HHS/United States
- K12 CA090628/CA/NCI NIH HHS/United States
- CA100634/CA/NCI NIH HHS/United States
- CA100080/CA/NCI NIH HHS/United States
- CA125614/CA/NCI NIH HHS/United States
- CA90628/CA/NCI NIH HHS/United States
- P50 CA100707/CA/NCI NIH HHS/United States
- R01 CA133966/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

