Leflunomide inhibition of BK virus replication in renal tubular epithelial cells
- PMID: 19955306
- PMCID: PMC2812362
- DOI: 10.1128/JVI.01737-09
Leflunomide inhibition of BK virus replication in renal tubular epithelial cells
Abstract
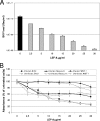
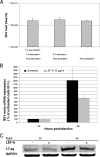
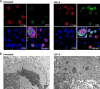
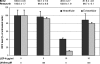
The immunomodulatory drug leflunomide is frequently used for treating polyomavirus-associated nephropathy, yet its antiviral mechanism is unclear. We characterized the effects of the active leflunomide metabolite A771726 (LEF-A) on the polyomavirus BK (BKV) life cycle in human renal tubular epithelial cells. LEF-A at 10 microg/ml reduced the extracellular BKV load by 90% (IC(90)) but with significant host cytostatic effects. BKV genome replication, late protein expression, and virion assembly and release were inhibited with visible disruption of the nuclear replication architecture. Both host cell and antiviral effects were largely reversed by uridine addition, implicating nonspecific pyrimidine depletion as the major anti-BKV mechanism of leflunomide.
Figures

References
-
- Awdishu, L., E. Sonbol, A. Feist, F. Lin, O. Alemi, R. Huang, and R. Pharmacy. 2009. Leflunomide delays recovery in kidney transplant recipients with BK viremia. American Transplant Congress 2009, abstr. L 503.
-
- Bansal, S., M. S. Lucia, and A. Wiseman. 2008. A case of polyomavirus-associated nephropathy presenting late after transplantation. Nat. Clin. Pract. Nephrol. 4:283-287. - PubMed
-
- Basse, G., C. Mengelle, N. Kamar, J. Guitard, D. Ribes, L. Esposito, and L. Rostaing. 2007. Prospective evaluation of BK virus DNAemia in renal transplant patients and their transplant outcome. Transplant. Proc. 39:84-87. - PubMed
-
- Baumann, P., S. Mandl-Weber, A. Volkl, C. Adam, I. Bumeder, F. Oduncu, and R. Schmidmaier. 2009. Dihydroorotate dehydrogenase inhibitor A771726 (leflunomide) induces apoptosis and diminishes proliferation of multiple myeloma cells. Mol. Cancer Ther. 8:366-375. - PubMed
-
- Bernhoff, E., T. J. Gutteberg, K. Sandvik, H. H. Hirsch, and C. H. Rinaldo. 2008. Cidofovir inhibits polyomavirus BK replication in human renal tubular cells downstream of viral early gene expression. Am. J. Transplant. 8:1413-1422. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

