Trial-to-trial variability of single cells in motor cortices is dynamically modified during visuomotor adaptation
- PMID: 19955356
- PMCID: PMC6665974
- DOI: 10.1523/JNEUROSCI.3011-09.2009
Trial-to-trial variability of single cells in motor cortices is dynamically modified during visuomotor adaptation
Abstract
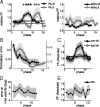
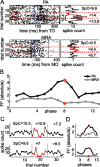
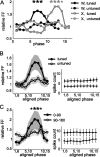
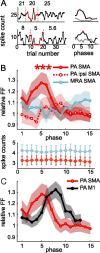
Neurons in all brain areas exhibit variability in their spiking activity. Although part of this variability can be considered as noise that is detrimental to information processing, recent findings indicate that variability can also be beneficial. In particular, it was suggested that variability in the motor system allows for exploration of possible motor states and therefore can facilitate learning and adaptation to new environments. Here, we provide evidence to support this idea by analyzing the variability of neurons in the primary motor cortex (M1) and in the supplementary motor area (SMA-proper) of monkeys adapting to new rotational visuomotor tasks. We found that trial-to-trial variability increased during learning and exhibited four main characteristics: (1) modulation occurred preferentially during a delay period when the target of movement was already known, but before movement onset; (2) variability returned to its initial levels toward the end of learning; (3) the increase in variability was more apparent in cells with preferred movement directions close to those experienced during learning; and (4) the increase in variability emerged at early phases of learning in the SMA, whereas in M1 behavior reached plateau levels of performance. These results are highly consistent with previous findings that showed similar trends in variability across a population of neurons. Together, the results strengthen the idea that single-cell variability can be much more than mere noise and may be an integral part of the underlying mechanism of sensorimotor learning.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
-
- Abeele S, Bock O. Mechanisms for sensorimotor adaptation to rotated visual input. Exp Brain Res. 2001;139:248–253. - PubMed
-
- Andersen RA, Buneo CA. Intentional maps in posterior parietal cortex. Annu Rev Neurosci. 2002;25:189–220. - PubMed
-
- Arieli A, Sterkin A, Grinvald A, Aertsen A. Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science. 1996a;273:1868–1871. - PubMed
-
- Arieli A, Sterkin A, Grinvald A, Aertsen A. Rehovot: The Grodetsky Center for Research of Higher Brain Functions; 1996b. Predicting cortical evoked responses in spite of their large variability—the impact of ongoing activity.
-
- Bach M, Kruger J. Correlated neuronal variability in monkey visual cortex revealed by a multi-microelectrode. Exp Brain Res. 1986;61:451–456. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
