Subthreshold activation of the superior colliculus drives saccade motor learning
- PMID: 19955374
- PMCID: PMC2828496
- DOI: 10.1523/JNEUROSCI.4296-09.2009
Subthreshold activation of the superior colliculus drives saccade motor learning
Abstract
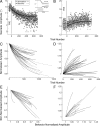
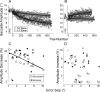
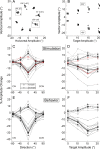
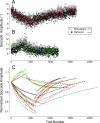
How the brain learns and maintains accurate precision movements is currently unknown. At times throughout life, rapid gaze shifts (saccades) become inaccurate, but the brain makes gradual adjustments so they again stop on target. Previously, we showed that complex spikes (CSs) in Purkinje cells of the oculomotor cerebellum report the direction and amplitude by which saccades are in error. Anatomical studies indicate that this error signal could originate in the superior colliculus (SC). Here, we deliver subthreshold electrical stimulation of the SC after the saccade lands to signal an apparent error. The size of saccades in the same direction as the simulated error gradually increase; those in the opposite direction decrease. The electrically adapted saccades endure after stimulation is discontinued, exhibit an adaptation field, can undergo changes in direction, and depend on error timing. These electrically induced adaptations were virtually identical with those produced by the visually induced adaptations that we report here for comparable visual errors in the same monkeys. Therefore, our experiments reveal that an additional role for the SC in the generation of saccades is to provide a vector error signal that drives dysmetric saccades to adapt. Moreover, the characteristics of the electrically induced adaptation reflect those of error-related CS activity in the oculomotor cerebellum, suggesting that CS activity serves as the learning signal. We speculate that CS activity may serve as the error signal that drives other kinds of motor learning as well.
Figures



References
-
- Albus JA. A theory of cerebellar function. Math Biosci. 1971;10:25–61.
-
- Anderson RW, Keller EL, Gandhi NJ, Das S. Two-dimensional saccade-related population activity in superior colliculus in monkey. J Neurophysiol. 1998;80:798–817. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
