Rho-associated kinase II (ROCKII) limits axonal growth after trauma within the adult mouse spinal cord
- PMID: 19955379
- PMCID: PMC2855556
- DOI: 10.1523/JNEUROSCI.4650-09.2009
Rho-associated kinase II (ROCKII) limits axonal growth after trauma within the adult mouse spinal cord
Abstract
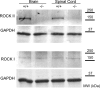
Rho GTPases are thought to mediate the action of several axonal growth inhibitors in the adult brain and spinal cord. RhoA has been targeted pharmacologically in both humans and animals to promote neurite outgrowth and functional recovery following CNS trauma. However, rat spinal cord injury studies suggest a complicated and partial benefit of inhibiting Rho or its downstream effector, Rho-associated kinase (ROCKII). This limited benefit may reflect inhibition of other kinases, poor access, or a minimal role of ROCKII in vivo. Therefore, we studied ROCKII mutant mice to probe this pathway genetically. ROCKII(-/-) dorsal root ganglion neurons are less sensitive to inhibition by Nogo protein or by chondroitin sulfate proteoglycan in vitro. We examined adult ROCKII(-/-) mice in two injury paradigms, cervical multilevel dorsal rhizotomy and midthoracic dorsal spinal cord hemisection. After dorsal root crush injury, the ROCKII(-/-) mice recovered use of the affected forepaw more quickly than did controls. Moreover, multiple classes of sensory axons regenerated across the dorsal root entry zone into the spinal cord of mice lacking ROCKII. After the spinal cord injury, ROCKII(-/-) mice showed enhanced local growth of raphespinal axons in the caudal spinal cord and corticospinal axons into the lesion site. Improved functional recovery was not observed by Basso Mouse Scale score following dorsal hemisection, likely due to developmental defects in the nervous system. Together, these findings demonstrate that the ROCKII gene product limits axonal growth after CNS trauma.
Figures









References
-
- Abad F, Feria M, Boada J. Chronic amitriptyline decreases autotomy following dorsal rhizotomy in rats. Neurosci Lett. 1989;99:187–190. - PubMed
-
- Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma. 2006;23:635–659. - PubMed
-
- Borisoff JF, Chan CC, Hiebert GW, Oschipok L, Robertson GS, Zamboni R, Steeves JD, Tetzlaff W. Suppression of Rho-kinase activity promotes axonal growth on inhibitory CNS substrates. Mol Cell Neurosci. 2003;22:405–416. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
