Activity of guard cell anion channel SLAC1 is controlled by drought-stress signaling kinase-phosphatase pair
- PMID: 19955405
- PMCID: PMC2795561
- DOI: 10.1073/pnas.0912021106
Activity of guard cell anion channel SLAC1 is controlled by drought-stress signaling kinase-phosphatase pair
Abstract
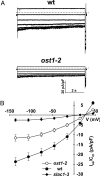
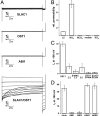
In response to drought stress the phytohormone ABA (abscisic acid) induces stomatal closure and, therein, activates guard cell anion channels in a calcium-dependent as well as-independent manner. Two key components of the ABA signaling pathway are the protein kinase OST1 (open stomata 1) and the protein phosphatase ABI1 (ABA insensitive 1). The recently identified guard cell anion channel SLAC1 appeared to be the key ion channel in this signaling pathway but remained electrically silent when expressed heterologously. Using split YFP assays, we identified OST1 as an interaction partner of SLAC1 and ABI1. Upon coexpression of SLAC1 with OST1 in Xenopus oocytes, SLAC1-related anion currents appeared similar to those observed in guard cells. Integration of ABI1 into the SLAC1/OST1 complex, however, prevented SLAC1 activation. Our studies demonstrate that SLAC1 represents the slow, deactivating, weak voltage-dependent anion channel of guard cells controlled by phosphorylation/dephosphorylation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Hetherington AM, Woodward FI. The role of stomata in sensing and driving environmental change. Nature. 2003;424:901–908. - PubMed
-
- Raschke K. Action of abscisic acid on guard cells. In: Zeiger GDF E, Cowan IR, editors. Stomatal Funktion. Stanford, CA: Stanford Univ Press; 1987. pp. 253–279.
-
- Roelfsema MR, Hedrich R. In the light of stomatal opening: New insights into “the Watergate”. New Phytol. 2005;167:665–691. - PubMed
-
- Schroeder JI, Kwak JM, Allen GJ. Guard cell abscisic acid signalling and engineering drought hardiness in plants. Nature. 2001;410:327–330. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

