Impairment of developmental stem cell-mediated striatal neurogenesis and pluripotency genes in a knock-in model of Huntington's disease
- PMID: 19955426
- PMCID: PMC2799796
- DOI: 10.1073/pnas.0912171106
Impairment of developmental stem cell-mediated striatal neurogenesis and pluripotency genes in a knock-in model of Huntington's disease
Abstract
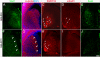
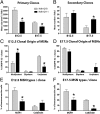
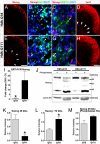
The pathogenesis of Huntington's disease (HD) remains elusive. The identification of increasingly early pathophysiological abnormalities in HD suggests the possibility that impairments of striatal medium spiny neuron (MSN) specification and maturation may underlie the etiology of HD. In fact, we demonstrate that HD knock-in (Hdh-Q111) mice exhibited delayed acquisition of early striatal cytoarchitecture with aberrant expression of progressive markers of MSN neurogenesis (Islet1, DARPP-32, mGluR1, and NeuN). Hdh-Q111 striatal progenitors also displayed delayed cell cycle exit between E13.5-15.5 (BrdU birth-dating) and an enhanced fraction of abnormal cycling cells in association with expansion of the pool of intermediate progenitors and over expression of the core pluripotency (PP) factor, Sox2. Clonal analysis further revealed that Hdh-Q111 neural stem cells (NSCs) displayed: impaired lineage restriction, reduced proliferative potential, enhanced late-stage self-renewal, and deregulated MSN subtype specification. Further, our analysis revealed that in addition to Sox2, the core PP factor, Nanog is expressed within the striatal generative and mantle regions, and in Hdh-Q111 embryos the fraction of Nanog-expressing MSN precursors was substantially increased. Moreover, compared to Hdh-Q18 embryos, the Hdh-Q111 striatal anlagen exhibited significantly higher levels of the essential PP cofactor, Stat3. These findings suggest that Sox2 and Nanog may play roles during a selective window of embryonic brain maturation, and alterations of these factors may, in part, be responsible for mediating the aberrant program of Hdh-Q111 striatal MSN specification and maturation. We propose that these HD-associated developmental abnormalities might compromise neuronal homeostasis and subsequently render MSNs more vulnerable to late life stressors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- U.S.-Venezuela Collaborative Research Project. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. The Huntington's disease collaborative research group. Cell. 1993;72:971–983. - PubMed
-
- Strong TV, et al. Widespread expression of the human and rat Huntington's disease gene in brain and nonneural tissues. Nat Genet. 1993;5:259–265. - PubMed
-
- Vonsattel JP, et al. Neuropathological classification of Huntington's disease. J Neuropathol Exp Neurol. 1985;44:559–577. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

