2,2,2-trichloroethanol activates a nonclassical potassium channel in cerebrovascular smooth muscle and dilates the middle cerebral artery
- PMID: 19955488
- PMCID: PMC2835435
- DOI: 10.1124/jpet.109.162313
2,2,2-trichloroethanol activates a nonclassical potassium channel in cerebrovascular smooth muscle and dilates the middle cerebral artery
Abstract
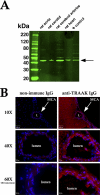
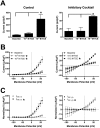
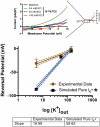
Trichloroacetaldehyde monohydrate [chloral hydrate (CH)] is a sedative/hypnotic that increases cerebral blood flow (CBF), and its active metabolite 2,2,2-trichloroethanol (TCE) is an agonist for the nonclassical two-pore domain K(+) (K(2P)) channels TREK-1 and TRAAK. We sought to determine whether TCE dilates cerebral arteries in vitro by activating nonclassical K(+) channels. TCE dilated pressurized and perfused rat middle cerebral arteries (MCAs) in a manner consistent with activation of nonclassical K(+) channels. Dilation to TCE was inhibited by elevated external K(+) but not by an inhibitory cocktail (IC) of classical K(+) channel blockers. Patch-clamp electrophysiology revealed that, in the presence of the IC, TCE increased whole-cell currents and hyperpolarized the membrane potential of isolated MCA smooth muscle cells. Heating increased TCE-sensitive currents, indicating that the activated channel was thermosensitive. Immunofluorescence in sections of the rat MCA demonstrated that, like TREK-1, TRAAK is expressed in the smooth muscle of cerebral arteries. Isoflurane did not, however, dilate the MCA, suggesting that TREK-1 was not functional. These data indicate that TCE activated a nonclassical K(+) channel with the characteristics of TRAAK in rat MCA smooth-muscle cells. Stimulation of K(+) channels such as TRAAK in cerebral arteries may therefore explain in part how CH/TCE increases CBF.
Figures
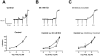


Similar articles
-
Evidence for two-pore domain potassium channels in rat cerebral arteries.Am J Physiol Heart Circ Physiol. 2006 Aug;291(2):H770-80. doi: 10.1152/ajpheart.01377.2005. Epub 2006 Mar 24. Am J Physiol Heart Circ Physiol. 2006. PMID: 16565299
-
Trichloroethanol enhances the activity of recombinant human TREK-1 and TRAAK channels.Neuropharmacology. 2004 Apr;46(5):750-60. doi: 10.1016/j.neuropharm.2003.11.023. Neuropharmacology. 2004. PMID: 14996553
-
cGMP does not activate two-pore domain K+ channels in cerebrovascular smooth muscle.Am J Physiol Heart Circ Physiol. 2009 Jun;296(6):H1774-80. doi: 10.1152/ajpheart.00082.2009. Epub 2009 Apr 10. Am J Physiol Heart Circ Physiol. 2009. PMID: 19363137 Free PMC article.
-
Cerebral artery K(ATP)- and K(Ca)-channel activity and contractility: changes with development.Am J Physiol Regul Integr Comp Physiol. 2000 Dec;279(6):R2004-14. doi: 10.1152/ajpregu.2000.279.6.R2004. Am J Physiol Regul Integr Comp Physiol. 2000. PMID: 11080063
-
Physiological roles and properties of potassium channels in arterial smooth muscle.Am J Physiol. 1995 Apr;268(4 Pt 1):C799-822. doi: 10.1152/ajpcell.1995.268.4.C799. Am J Physiol. 1995. PMID: 7733230 Review.
Cited by
-
Restoration of Endothelial Function in Pparα (-/-) Mice by Tempol.PPAR Res. 2015;2015:728494. doi: 10.1155/2015/728494. Epub 2015 Nov 15. PPAR Res. 2015. PMID: 26649033 Free PMC article.
-
TREK-1 Channel Expression in Smooth Muscle as a Target for Regulating Murine Intestinal Contractility: Therapeutic Implications for Motility Disorders.Front Physiol. 2018 Mar 6;9:157. doi: 10.3389/fphys.2018.00157. eCollection 2018. Front Physiol. 2018. PMID: 29563879 Free PMC article.
-
Development of BOLD signal hemodynamic responses in the human brain.Neuroimage. 2012 Nov 1;63(2):663-73. doi: 10.1016/j.neuroimage.2012.06.054. Epub 2012 Jul 6. Neuroimage. 2012. PMID: 22776460 Free PMC article.
-
PPARα-Independent Arterial Smooth Muscle Relaxant Effects of PPARα Agonists.PPAR Res. 2012;2012:302495. doi: 10.1155/2012/302495. Epub 2012 Sep 11. PPAR Res. 2012. PMID: 23008696 Free PMC article.
-
Store-operated calcium entry is present in HL-1 cardiomyocytes and contributes to resting calcium.Biochem Biophys Res Commun. 2011 Dec 9;416(1-2):45-50. doi: 10.1016/j.bbrc.2011.10.133. Epub 2011 Nov 6. Biochem Biophys Res Commun. 2011. PMID: 22079292 Free PMC article.
References
-
- Andresen JJ, Shafi NI, Durante W, Bryan RM., Jr (2006) Effects of carbon monoxide and heme oxygenase inhibitors in cerebral vessels of rats and mice. Am J Physiol Heart Circ Physiol 291:H223–H230 - PubMed
-
- Beland FA. (1999) NTP technical report on the toxicity and metabolism studies of chloral hydrate (CAS No. 302-17-0). Administered by gavage to F344/N rats and B6C3F1 mice. Toxic Rep Ser 1–66, A61–E67 - PubMed
-
- Blondeau N, Pétrault O, Manta S, Giordanengo V, Gounon P, Bordet R, Lazdunski M, Heurteaux C. (2007) Polyunsaturated fatty acids are cerebral vasodilators via the TREK-1 potassium channel. Circ Res 101:176–184 - PubMed
-
- Bryan RM, Jr, You J, Phillips SC, Andresen JJ, Lloyd EE, Rogers PA, Dryer SE, Marrelli SP. (2006) Evidence for two-pore domain potassium channels in rat cerebral arteries. Am J Physiol Heart Circ Physiol 291:H770–H780 - PubMed
-
- Cabana BE, Gessner PK. (1970) The kinetics of chloral hydrate metabolism in mice and the effect thereon of ethanol. J Pharmacol Exp Ther 174:260–275 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

