TRAMP complex enhances RNA degradation by the nuclear exosome component Rrp6
- PMID: 19955569
- PMCID: PMC2823493
- DOI: 10.1074/jbc.M109.058396
TRAMP complex enhances RNA degradation by the nuclear exosome component Rrp6
Abstract
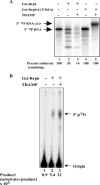
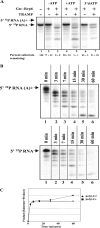
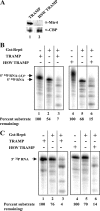
The RNA-processing exosome contains ribonucleases that degrade aberrant RNAs in archael and eukaryotic cells. In Saccharomyces cerevisiae, the nuclear/nucleolar 3'-5' exoribonuclease Rrp6 distinguishes the nuclear exosome from the cytoplasmic exosome. In vivo, the TRAMP complex enhances the ability of the nuclear exosome to destroy some aberrant RNAs. Previous reports showed that purified TRAMP enhanced RNA degradation by the nuclear exosome in vitro. However, the exoribonucleolytic component(s) of the nuclear exosome enhanced by TRAMP remain unidentified. We show that TRAMP does not significantly enhance RNA degradation by purified exosomes lacking Rrp6 in vitro, suggesting that TRAMP activation experiments with nuclear exosome preparations reflect, in part, effects on the activity of Rrp6. Consistent with this, we show that incubation of purified TRAMP with recombinant Rrp6 results in a 10-fold enhancement of the rate of RNA degradation. This increased activity results from enhancement of the hydrolytic activity of Rrp6 because TRAMP cannot enhance the activity of an Rrp6 mutant lacking a key amino acid side chain in its active site. We observed no ATP or polyadenylation dependence for the enhancement of Rrp6 activity by TRAMP, suggesting that neither the poly(A) polymerase activity of Trf4 nor the helicase activity of Mtr4 plays a role in the enhancement. These findings identify TRAMP as an exosome-independent enhancer of Rrp6 activity.
Figures

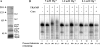

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

