TGF-beta1-induced expression of human Mdm2 correlates with late-stage metastatic breast cancer
- PMID: 19955655
- PMCID: PMC2798681
- DOI: 10.1172/JCI39194
TGF-beta1-induced expression of human Mdm2 correlates with late-stage metastatic breast cancer
Abstract
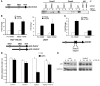
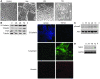
The E3 ubiquitin ligase human murine double minute (HDM2) is overexpressed in 40%-80% of late-stage metastatic cancers in the absence of gene amplification. Hdm2 regulates p53 stability via ubiquitination and has also been implicated in altering the sensitivity of cells to TGF-beta1. Whether TGF-beta1 signaling induces Hdm2 expression leading to HDM2-mediated destabilization of p53 has not been investigated. In this study, we report that TGF-beta1-activated SMA- and MAD3 (Smad3/4) transcription factors specifically bound to the second promoter region of HDM2, leading to increased HDM2 protein expression and destabilization of p53 in human cancer cell lines. Additionally, TGF-beta1 expression led to Smad3 activation and murine double minute 2 (Mdm2) expression in murine mammary epithelial cells during epithelial-to-mesenchymal transition (EMT). Furthermore, histological analyses of human breast cancer samples demonstrated that approximately 65% of late-stage carcinomas were positive for activated Smad3 and HDM2, indicating a strong correlation between TGF-beta1-mediated induction of HDM2 and late-stage tumor progression. Identification of Hdm2 as a downstream target of TGF-beta1 represents a critical prosurvival mechanism in cancer progression and provides another point for therapeutic intervention in late-stage cancer.
Figures


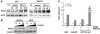


References
-
- Gold LI. The role for transforming growth factor-beta (TGF-beta) in human cancer. Crit Rev Oncog. 1999;10(4):303–360. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

