Deletion of Fas in adipocytes relieves adipose tissue inflammation and hepatic manifestations of obesity in mice
- PMID: 19955656
- PMCID: PMC2798678
- DOI: 10.1172/JCI38388
Deletion of Fas in adipocytes relieves adipose tissue inflammation and hepatic manifestations of obesity in mice
Abstract
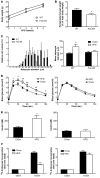
Adipose tissue inflammation is linked to the pathogenesis of insulin resistance. In addition to exerting death-promoting effects, the death receptor Fas (also known as CD95) can activate inflammatory pathways in several cell lines and tissues, although little is known about the metabolic consequence of Fas activation in adipose tissue. We therefore sought to investigate the contribution of Fas in adipocytes to obesity-associated metabolic dysregulation. Fas expression was markedly increased in the adipocytes of common genetic and diet-induced mouse models of obesity and insulin resistance, as well as in the adipose tissue of obese and type 2 diabetic patients. Mice with Fas deficiency either in all cells or specifically in adipocytes (the latter are referred to herein as AFasKO mice) were protected from deterioration of glucose homeostasis induced by high-fat diet (HFD). Adipocytes in AFasKO mice were more insulin sensitive than those in wild-type mice, and mRNA levels of proinflammatory factors were reduced in white adipose tissue. Moreover, AFasKO mice were protected against hepatic steatosis and were more insulin sensitive, both at the whole-body level and in the liver. Thus, Fas in adipocytes contributes to adipose tissue inflammation, hepatic steatosis, and insulin resistance induced by obesity and may constitute a potential therapeutic target for the treatment of insulin resistance and type 2 diabetes.
Figures


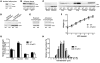




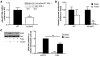
Comment in
-
The chicken or the egg: adipocytes and hepatic insulin resistance.Hepatology. 2010 Mar;51(3):1076-9. doi: 10.1002/hep.23580. Hepatology. 2010. PMID: 20198702 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

