Exercise and insulin: Convergence or divergence at AS160 and TBC1D1?
- PMID: 19955868
- PMCID: PMC2789346
- DOI: 10.1097/JES.0b013e3181b7b7c5
Exercise and insulin: Convergence or divergence at AS160 and TBC1D1?
Abstract
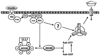
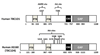
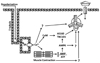
Akt substrate of 160 kDa (called AS160 or TBC1D4) and TBC1D1, Rab GTPase-activating proteins that regulate glucose transport, become phosphorylated with exercise or insulin stimulation. Evidence suggests that this convergence may prove to be imperfect, and each stimulus will produce a unique phosphosignature, providing a plausible mechanism for their apparently unique and overlapping roles in exercise- and insulin-stimulated glucose transport.
Figures

Comment in
-
Exercise and insulin - understanding the molecular interactions.Exerc Sport Sci Rev. 2009 Oct;37(4):156. doi: 10.1097/JES.0b013e3181b95462. Exerc Sport Sci Rev. 2009. PMID: 19955863 No abstract available.
References
-
- Arias EB, Kim J, Cartee GD. Prolonged incubation in PUGNAc results in increased protein O-Linked glycosylation and insulin resistance in rat skeletal muscle. Diabetes. 2004;53:921–930. - PubMed
-
- Arias EB, Kim J, Funai K, Cartee GD. Prior exercise increases phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle. Am J Physiol Endocrinol Metab. 2007;292:E1191–E1200. - PubMed
-
- Bruss MD, Arias EB, Lienhard GE, Cartee GD. Increased phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle in response to insulin or contractile activity. Diabetes. 2005;54:41–50. - PubMed
-
- Cartee GD, Wojtaszewski JF. Role of Akt substrate of 160 kDa in insulin-stimulated and contraction-stimulated glucose transport. Appl Physiol Nutr Metab. 2007;32:557–566. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

