Multiquantal release underlies the distribution of synaptic efficacies in the neocortex
- PMID: 19956403
- PMCID: PMC2786302
- DOI: 10.3389/neuro.10.027.2009
Multiquantal release underlies the distribution of synaptic efficacies in the neocortex
Abstract
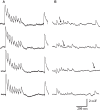
Inter-pyramidal synaptic connections are characterized by a wide range of EPSP amplitudes. Although repeatedly observed at different brain regions and across layers, little is known about the synaptic characteristics that contribute to this wide range. In particular, the range could potentially be accounted for by differences in all three parameters of the quantal model of synaptic transmission, i.e. the number of release sites, release probability and quantal size. Here, we present a rigorous statistical analysis of the transmission properties of excitatory synaptic connections between layer-5 pyramidal neurons of the somato-sensory cortex. Our central finding is that the EPSP amplitude is strongly correlated with the number of estimated release sites, but not with the release probability or quantal size. In addition, we found that the number of release sites can be more than an order of magnitude higher than the typical number of synaptic contacts for this type of connection. Our findings indicate that transmission at stronger synaptic connections is mediated by multiquantal release from their synaptic contacts. We propose that modulating the number of release sites could be an important mechanism in regulating neocortical synaptic transmission.
Keywords: neocortex; quantal analysis; short-term depression; synaptic transmission.
Figures





References
LinkOut - more resources
Full Text Sources

