Neuroendocrine differentiation in prostate cancer
- PMID: 19956427
- PMCID: PMC2776313
Neuroendocrine differentiation in prostate cancer
Abstract
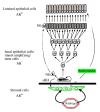
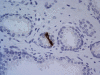
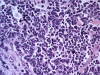
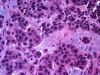
As any organ in the body human prostate is composed of many different types of cells as well as extracellular components. During prostate development, reciprocal cellular interactions between stromal cells and prostate epithelial cells ultimately lead to the development of a mature prostate. Normal prostate is composed of repeating cellular units that contain stromal and epithelial compartments. The epithelial compartment contains luminal epithelial cells, basal cells and a minor component of neuroendocrine cells whose function may be to regulate the growth, differentiation and secretory function of the prostate gland. Neuroendocrine cells are also evident in prostate cancer and numerous studies showed that its number increases in high grade and high stage tumors, particularly in hormonally treated and hormone-refractory (androgen-independent) prostate cancer. Although androgen withdrawal reduces the secretion of the andromedins from the prostate stromal cells that are critical for the survival for prostate epithelial cells, there is clear evidence that androgen receptor is also required for the tumorigenesis of human prostate cancer, and therefore androgen deprivation therapy likely works through inhibition of androgen receptor in the prostate epithelium. Because neuroendocrine cells lack androgen receptor and are likely androgen-independent, it is conceivable that hormonal therapy for advanced/metastatic prostate cancer, which consists of inhibiting androgen production and/or blocking androgen receptor function, will not eliminate neuroendocrine cancer cells. Instead, these cells may be enriched after the therapy and they may establish paracrine networks to stimulate androgen-independent proliferation of prostate cancer, leading to tumor recurrence. In this article, we will review the known functions of the neuroendocrine cells in prostate cancer, including stimulation of cancer proliferation and invasion, apoptosis resistance and angiogenesis as well as molecular pathways involved in neuroendocrine differentiation.
Keywords: Prostate cancer; hormonal therapy; neuroendocrine.
Figures
References
-
- Isaacs JT, Coffey DS. Etiology and disease process of benign prostatic hyperplasia. Prostate Suppl. 1989;2:33–50. - PubMed
-
- Bonkhoff H, Stein U, Remberger K. Multidirectional differentiation in the normal, hyperplastic, and neoplastic human prostate: simultaneous demonstration of cell-specific epithelial markers. Hum Pathol. 1994;25:42–46. - PubMed
-
- Bonkhoff H, Remberger K. Differentiation pathways and histogenetic aspects of normal and abnormal prostatic growth: a stem cell model. Prostate. 1996;28:98–106. - PubMed
-
- Qiu Y, Robinson D, Pretlow TG, Kung HJ. Etk/Bmx, a tyrosine kinase with a pleckstrin-homology domain, is an effector of phosphatidylinositol 3′-kinase and is involved in interleukin 6-induced neuroendocrine differentiation of prostate cancer cells. Proc Natl Acad Sci U S A. 1998;95:3644–3649. - PMC - PubMed
-
- van Leenders G, Dijkman H, Hulsbergen-van de Kaa C, Ruiter D, Schalken J. Demonstration of intermediate cells during human prostate epithelial differentiation in situ and in vitro using triple-staining confocal scanning microscopy. Lab Invest. 2000;80:1251–1258. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
