Angiotensin-converting enzyme levels and activity in Alzheimer's disease: differences in brain and CSF ACE and association with ACE1 genotypes
- PMID: 19956428
- PMCID: PMC2776311
Angiotensin-converting enzyme levels and activity in Alzheimer's disease: differences in brain and CSF ACE and association with ACE1 genotypes
Abstract
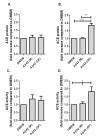
Angiotensin-converting enzyme (ACE) has been implicated in Alzheimer's disease (AD): ACE1 variations influence plasma ACE and risk of AD, and ACE is increased in AD brain. We measured frontal ACE level and activity in 89 AD and 51 control brains, and post-mortem CSF from 101 cases and 19 controls. Neuron-specific enolase (NSE) level and Braak stage were used to indicate neuronal preservation and disease progression. We genotyped the common ACE insertion/deletion polymorphism, rs4343, rs1800764 and rs4921. ACE activity was elevated in AD and correlated with Braak stage. Crude ACE levels were unchanged but adjustment for NSE suggested increased neuronal ACE production with Braak stage. Exposing SH-SY-5Y neurons to oligomeric Abeta1-42 increased ACE level and activity, suggesting Abeta may upregulate ACE in AD. In CSF, ACE level but not activity was reduced in AD. ACE1 genotype did not predict ACE level or activity in brain or CSF. ACE activity and neuronal production increase in AD brain, possibly in response to Abeta. Peripheral measurements do not reflect ACE activity in the brain.
Keywords: ACE1; Alzheimer's disease; Angiotensin-converting enzyme; Braak stage; cerebrospinal fluid; enzyme activity; neuron-specific enolase.
Figures




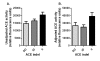

References
-
- Turner AJ, Hooper NM. The angiotensin-converting enzyme gene family: genomics and pharmacology. Trends Pharmacol Sci. 2002;23:177–183. - PubMed
-
- Parkin ET, Turner AJ, Hooper NM. Secretase-mediated cell surface shedding of the angiotensin-converting enzyme. Protein Pept Lett. 2004;11:423–432. - PubMed
-
- Sayed-Tabatabaei FA, Oostra BA, Isaacs A, van Duijn CM, Witteman JCM. ACE Polymorphisms. Circ Res. 2006;98:1123–1133. - PubMed
-
- Kehoe PG, Wilcock GK. Is inhibition of the renin-angiotensin system a new treatment option for Alzheimer's disease? Lancet Neurol. 2007;6:373–378. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
