Modulation of NFAT-5, an outlying member of the NFAT family, in human keratinocytes and skin
- PMID: 19956430
- PMCID: PMC2776318
Modulation of NFAT-5, an outlying member of the NFAT family, in human keratinocytes and skin
Abstract
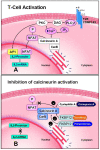
Background: Cyclosporin A (CsA) and tacrolimus block T cell activation by inhibiting the phosphatase calcineurin and preventing translocation from the cytoplasm to the nucleus of the transcription factor Nuclear Factor of Activated T cells (NFAT). NFAT compose a family of transcription factors that are turned on during T cell activation.
Aims: To study the expression of NFAT-5 mRNA and protein in normal human keratinocytes and to investigate the cellular and subcellular pattern of expression of NFAT-5 in normal human skin and psoriasis, and analyze effects of different agonists and ultraviolet radiation on NFAT-5 in normal human skin.
Methods: Tissue cultures, Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR), Western analysis, immunostaining, confocal microscopy.
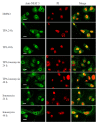
Results: Sequencing of RT-PCR products confirmed the identity of the product that showed 100 % homology with the predicted NFAT-5 sequence. anti-NFAT-5 mainly detected a single band in cultured keratinocytes and dermal fibroblasts using Western analysis. Immunohistochemistry showed that epidermal keratinocytes and dermal fibroblasts in normal human and psoriatic skin express NFAT-5. NFAT-5 showed predominantly nuclear localization in epidermal keratinocytes and dermal fibroblasts within five normal adult skin biopsies. Our data also suggest that UV irradiation reduces NFAT-5 nuclear localization within the epidermis. Unlike NFAT 1-4, NFAT-5/TonEBP was localized to both nucleus and cytoplasm of cultured keratinocytes. Cyclosporin A induces nuclear membrane translocation of NFAT-5 in cultured keratinocytes and raffinose (a hypertonicity inducing agent) induces more nuclear localization of NFAT-5 compared to untreated cells. In addition, differentiation-promoting agonists that induce sustained rise in intracellular calcium did not result in changes in NFAT-5 localization in cultured keratinocytes.
Conclusion: These studies provide the first observation of expression of NFAT-5/TonEBP mRNA protein in cultured keratinocytes and dermal fibroblasts and possible functional regulation in cultured keratinocytes. CsA and raffinose effects on NFAT-5/TonEBP in cultured keratinocytes suggest diverse intracellular signaling pathways for NFAT-5/TonEBP in these cells, and that NFAT-5/TonEBP might function to translate different extracellular stimuli into appropriate functional responses.
Keywords: Cyclosporin A (CsA); Human keratinocytes; Hypertonicity; NFAT-5 (TonEBP); UVR; psoriasis.
Figures






Similar articles
-
Localization of calcineurin/NFAT in human skin and psoriasis and inhibition of calcineurin/NFAT activation in human keratinocytes by cyclosporin A.J Invest Dermatol. 2002 May;118(5):779-88. doi: 10.1046/j.1523-1747.2002.01709.x. J Invest Dermatol. 2002. PMID: 11982754
-
Expression, localisation and functional activation of NFAT-2 in normal human skin, psoriasis, and cultured keratocytes.Int J Clin Exp Med. 2009 Jun 18;2(2):176-92. Int J Clin Exp Med. 2009. PMID: 19684889 Free PMC article.
-
Calcineurin/NFAT-dependent regulation of 230-kDa bullous pemphigoid antigen (BPAG1) gene expression in normal human epidermal keratinocytes.J Dermatol Sci. 2008 Jul;51(1):45-51. doi: 10.1016/j.jdermsci.2008.01.006. Epub 2008 Mar 18. J Dermatol Sci. 2008. PMID: 18353617
-
A preliminary examination of the role of NFAT 3 in human skin, cultured keratocytes and dermal fibroblasts.J Cutan Pathol. 2010 Sep;37(9):e21-36. doi: 10.1111/j.1600-0560.2009.01313.x. J Cutan Pathol. 2010. Retraction in: J Cutan Pathol. 2011 Jul;38(7):600. doi: 10.1111/j.1600-0560.2011.01735.x. PMID: 20653821 Retracted.
-
NFAT regulates induction of COX-2 and apoptosis of keratinocytes in response to ultraviolet radiation exposure.FASEB J. 2008 Dec;22(12):4218-27. doi: 10.1096/fj.08-113076. Epub 2008 Aug 15. FASEB J. 2008. PMID: 18708588 Free PMC article.
Cited by
-
Tacrolimus reverses pemphigus vulgaris serum-induced depletion of desmoglein in HaCaT cells via inhibition of heat shock protein 27 phosphorylation.BMC Immunol. 2023 Nov 8;24(1):43. doi: 10.1186/s12865-023-00582-z. BMC Immunol. 2023. PMID: 37940861 Free PMC article.
-
Downregulation of NFAT2 promotes melanogenesis in B16 melanoma cells.Anat Cell Biol. 2010 Dec;43(4):303-9. doi: 10.5115/acb.2010.43.4.303. Epub 2010 Dec 31. Anat Cell Biol. 2010. PMID: 21267404 Free PMC article.
References
-
- Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–747. - PubMed
-
- Pan S, Tsuruta R, Masuda ES, Imamura R, Bazan F, Arai K, Arai N, Miyatake S. NFATz: a novel rel similarity domain containing protein. Biochem Biophys Res Commun. 2000;272:765–776. - PubMed
-
- Ko BC, Turck CW, Lee KW, Yang Y, Chung SS. Purification, identification, and characterization of an osmotic response element binding protein. Biochem Biophys Res Commun. 2000;270:52–61. - PubMed
-
- Trama J, Lu Q, Hawley RG, Ho SN. The NFAT-related protein NFATL1 (TonEBP/NFAT5) is induced upon T cell activation in a calcineurin-dependent manner. J Immunol. 2000;165:4884–4894. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
